A rare adverse effect in inebilizumab therapy for neuromyelitis optica spectrum disorder: a case report
- PMID: 39072007
- PMCID: PMC11282551
- DOI: 10.1177/17562864241258787
A rare adverse effect in inebilizumab therapy for neuromyelitis optica spectrum disorder: a case report
Abstract
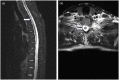
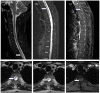
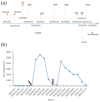
Inebilizumab is one of the monoclonal antibodies approved as maintenance therapy for aquaporin-4 immunoglobulin G-seropositive neuromyelitis optica spectrum disorder (NMOSD). It is a humanized monoclonal antibody targeting cluster of differentiation 19 (CD19). Common adverse reactions include urinary tract infections, nasopharyngitis, arthralgia, infusion reactions, headaches and a decrease in immunoglobulin levels. Here, we present a case of an NMOSD patient who experienced transient hyperCKaemia after the use of inebilizumab. The adverse reactions of this very rare monoclonal antibody drug improved after discontinuation.
Keywords: adverse effect; case report; hyperCKaemia; inebilizumab; neuromyelitis optica spectrum disorder.
© The Author(s), 2024.
Figures
Similar articles
-
Inebilizumab: A Review in Neuromyelitis Optica Spectrum Disorder.CNS Drugs. 2022 Oct;36(10):1133-1141. doi: 10.1007/s40263-022-00949-7. Epub 2022 Sep 7. CNS Drugs. 2022. PMID: 36070074 Free PMC article. Review.
-
Safety and efficacy of inebilizumab for the treatment of neuromyelitis optica spectrum disorder: end-of-study results from the open-label period of the N-MOmentum trial.Lancet Neurol. 2024 Jun;23(6):588-602. doi: 10.1016/S1474-4422(24)00077-2. Lancet Neurol. 2024. PMID: 38760098 Clinical Trial.
-
Inebilizumab in AQP4-Ab-positive neuromyelitis optica spectrum disorder.Drugs Today (Barc). 2021 May;57(5):321-336. doi: 10.1358/dot.2021.57.5.3265453. Drugs Today (Barc). 2021. PMID: 34061127 Review.
-
Case report: Transition from anti-CD20 therapy to inebilizumab for 14 cases of neuromyelitis optica spectrum disorder.Front Neurol. 2024 Apr 16;15:1352779. doi: 10.3389/fneur.2024.1352779. eCollection 2024. Front Neurol. 2024. PMID: 38689876 Free PMC article.
-
Inebilizumab for treatment of neuromyelitis optica spectrum disorder.Neurodegener Dis Manag. 2021 Oct;11(5):341-352. doi: 10.2217/nmt-2021-0017. Epub 2021 Sep 6. Neurodegener Dis Manag. 2021. PMID: 34486379
References
-
- Cree BAC, Bennett JL, Kim HJ, et al.. Inebilizumab for the treatment of neuromyelitis optica spectrum disorder (N-MOmentum): a double-blind, randomised placebo-controlled phase 2/3 trial. Lancet 2019; 394: 1352–1363. - PubMed
-
- Liu SW, Velez NF, Lam C, et al.. Dermatomyositis induced by anti-tumor necrosis factor in a patient with juvenile idiopathic arthritis. JAMA Dermatol 2013; 149: 1204–1208. - PubMed
-
- He D, Li Y, Dai Q, et al.. Myopathy associated with neuromyelitis optica spectrum disorders. Int J Neurosci 2016; 126: 863–866. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

