Comparison of UVC Sensitivity and Dectin-2 Expression Between Malignant and Non-malignant Cells
- PMID: 36099100
- PMCID: PMC9463890
- DOI: 10.21873/invivo.12937
Comparison of UVC Sensitivity and Dectin-2 Expression Between Malignant and Non-malignant Cells
Abstract
Background/aim: Rapid spread of COVID-19 resulted in the revision of the value of ultraviolet C (UVC) sterilization in working spaces. This study aimed at investigating the UVC sensitivity of eighteen malignant and nonmalignant cell lines, the protective activity of sodium ascorbate against UVC, and whether Dectin-2 is involved in UVC sensitivity.
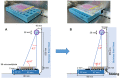
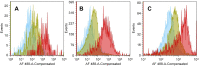
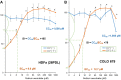
Materials and methods: Various cell lines were exposed to UVC for 3 min, and cell viability was determined using the MTT assay. Anti-UV activity was determined as the ratio of 50% cytotoxic concentration (determined with unirradiated cells) to 50% effective concentration (that restored half of the UV-induced loss of viability). Dectin-2 expression was quantified using flow cytometry.
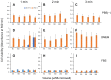
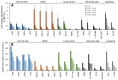
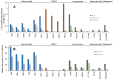
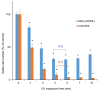
Results: The use of culture medium rather than phosphate-buffered saline is recommended as irradiation solution, since several cells are easily detached during irradiation in phosphate-buffered saline. Oral squamous cell carcinoma cell lines showed the highest UV sensitivity, followed by neuroblastoma, glioblastoma, leukemia, melanoma, lung carcinoma cells, and normal oral and dermal fibroblasts. Human dermal fibroblasts were more resistant than melanoma cell lines; however, both expressed Dectin-2. Sodium ascorbate at micromolar concentrations eliminated the cytotoxicity of UVC in these cell lines.
Conclusion: Normal cells are generally UVC-resistant compared to corresponding malignant cells, which have higher growth potential. Dectin-2 protein expression itself may not be determinant of UVC sensitivity.
Keywords: Dectin-2; UVC sensitivity; dermal fibroblast; malignancy; melanoma; protection; sodium ascorbate.
Copyright © 2022, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Figures

Similar articles
-
UVC-Protective Activity of Lemongrass Among 12 Fat-soluble Herbal Extracts: Rapid Decay Due to Cytotoxicity.In Vivo. 2023 Nov-Dec;37(6):2464-2472. doi: 10.21873/invivo.13353. In Vivo. 2023. PMID: 37905640 Free PMC article.
-
Comprehensive Study of Anti-UVC Activity and Cytotoxicity of Hot-water Soluble Herb Extracts.In Vivo. 2023 Jul-Aug;37(4):1540-1551. doi: 10.21873/invivo.13239. In Vivo. 2023. PMID: 37369486 Free PMC article.
-
Prominent Anti-UVC Activity of Lignin Degradation Products.In Vivo. 2022 Nov-Dec;36(6):2689-2699. doi: 10.21873/invivo.13004. In Vivo. 2022. PMID: 36309360 Free PMC article.
-
Role of UVA in the pathogenesis of melanoma and non-melanoma skin cancer. A short review.Photodermatol Photoimmunol Photomed. 1999 Dec;15(6):212-6. doi: 10.1111/j.1600-0781.1999.tb00090.x. Photodermatol Photoimmunol Photomed. 1999. PMID: 10599968 Review.
-
Final report of the safety assessment of L-Ascorbic Acid, Calcium Ascorbate, Magnesium Ascorbate, Magnesium Ascorbyl Phosphate, Sodium Ascorbate, and Sodium Ascorbyl Phosphate as used in cosmetics.Int J Toxicol. 2005;24 Suppl 2:51-111. doi: 10.1080/10915810590953851. Int J Toxicol. 2005. PMID: 16154915 Review.
Cited by
-
UVC-Protective Activity of Lemongrass Among 12 Fat-soluble Herbal Extracts: Rapid Decay Due to Cytotoxicity.In Vivo. 2023 Nov-Dec;37(6):2464-2472. doi: 10.21873/invivo.13353. In Vivo. 2023. PMID: 37905640 Free PMC article.
-
Comprehensive Study of Anti-UVC Activity and Cytotoxicity of Hot-water Soluble Herb Extracts.In Vivo. 2023 Jul-Aug;37(4):1540-1551. doi: 10.21873/invivo.13239. In Vivo. 2023. PMID: 37369486 Free PMC article.
-
Prominent Anti-UVC Activity of Lignin Degradation Products.In Vivo. 2022 Nov-Dec;36(6):2689-2699. doi: 10.21873/invivo.13004. In Vivo. 2022. PMID: 36309360 Free PMC article.
References
-
- Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. - DOI - PMC - PubMed
-
- Harfoot R, Yung DBY, Anderson WA, Wild CEK, Coetzee N, Hernández LC, Lawley B, Pletzer D, Derraik JGB, Anderson YC, Quiñones-Mateu ME. Ultraviolet-C irradiation, heat, and storage as potential methods of inactivating SARS-CoV-2 and bacterial pathogens on filtering facepiece respirators. Pathogens. 2022;11(1):83. doi: 10.3390/pathogens11010083. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
