Pseudomonas aeruginosa Alters Staphylococcus aureus Sensitivity to Vancomycin in a Biofilm Model of Cystic Fibrosis Infection
- PMID: 28720732
- PMCID: PMC5516255
- DOI: 10.1128/mBio.00873-17
Pseudomonas aeruginosa Alters Staphylococcus aureus Sensitivity to Vancomycin in a Biofilm Model of Cystic Fibrosis Infection
Abstract
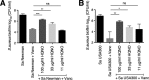
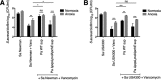
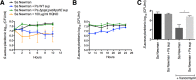
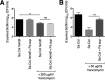
The airways of cystic fibrosis (CF) patients have thick mucus, which fosters chronic, polymicrobial infections. Pseudomonas aeruginosa and Staphylococcus aureus are two of the most prevalent respiratory pathogens in CF patients. In this study, we tested whether P. aeruginosa influences the susceptibility of S. aureus to frontline antibiotics used to treat CF lung infections. Using our in vitro coculture model, we observed that addition of P. aeruginosa supernatants to S. aureus biofilms grown either on epithelial cells or on plastic significantly decreased the susceptibility of S. aureus to vancomycin. Mutant analyses showed that 2-n-heptyl-4-hydroxyquinoline N-oxide (HQNO), a component of the P. aeruginosa Pseudomonas quinolone signal (PQS) system, protects S. aureus from the antimicrobial activity of vancomycin. Similarly, the siderophores pyoverdine and pyochelin also contribute to the ability of P. aeruginosa to protect S. aureus from vancomycin, as did growth under anoxia. Under our experimental conditions, HQNO, P. aeruginosa supernatant, and growth under anoxia decreased S. aureus growth, likely explaining why this cell wall-targeting antibiotic is less effective. P. aeruginosa supernatant did not confer additional protection to slow-growing S. aureus small colony variants. Importantly, P. aeruginosa supernatant protects S. aureus from other inhibitors of cell wall synthesis as well as protein synthesis-targeting antibiotics in an HQNO- and siderophore-dependent manner. We propose a model whereby P. aeruginosa causes S. aureus to shift to fermentative growth when these organisms are grown in coculture, leading to reduction in S. aureus growth and decreased susceptibility to antibiotics targeting cell wall and protein synthesis.IMPORTANCE Cystic fibrosis (CF) lung infections are chronic and difficult to eradicate. Pseudomonas aeruginosa and Staphylococcus aureus are two of the most prevalent respiratory pathogens in CF patients and are associated with poor patient outcomes. Both organisms adopt a biofilm mode of growth, which contributes to high tolerance to antibiotic treatment and the recalcitrant nature of these infections. Here, we show that P. aeruginosa exoproducts decrease the sensitivity of S. aureus biofilm and planktonic populations to vancomycin, a frontline antibiotic used to treat methicillin-resistant S. aureus in CF patients. P. aeruginosa also protects S. aureus from other cell wall-active antibiotics as well as various classes of protein synthesis inhibitors. Thus, interspecies interactions can have dramatic and unexpected consequences on antibiotic sensitivity. This study underscores the potential impact of interspecies interactions on antibiotic efficacy in the context of complex, polymicrobial infections.
Keywords: Pseudomonas; Staphylococcus aureus; antibiotic tolerance; biofilms; cystic fibrosis; polymicrobial.
Copyright © 2017 Orazi and O’Toole.
Figures
Similar articles
-
Pseudomonas aeruginosa Increases the Sensitivity of Biofilm-Grown Staphylococcus aureus to Membrane-Targeting Antiseptics and Antibiotics.mBio. 2019 Jul 30;10(4):e01501-19. doi: 10.1128/mBio.01501-19. mBio. 2019. PMID: 31363032 Free PMC article.
-
Pseudomonas aeruginosa PA14 Enhances the Efficacy of Norfloxacin against Staphylococcus aureus Newman Biofilms.J Bacteriol. 2020 Aug 25;202(18):e00159-20. doi: 10.1128/JB.00159-20. Print 2020 Aug 25. J Bacteriol. 2020. PMID: 32661077 Free PMC article.
-
Exogenous Alginate Protects Staphylococcus aureus from Killing by Pseudomonas aeruginosa.J Bacteriol. 2020 Mar 26;202(8):e00559-19. doi: 10.1128/JB.00559-19. Print 2020 Mar 26. J Bacteriol. 2020. PMID: 31792010 Free PMC article.
-
Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis.Cochrane Database Syst Rev. 2015 Mar 5;(3):CD009528. doi: 10.1002/14651858.CD009528.pub3. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2017 Oct 05;10:CD009528. doi: 10.1002/14651858.CD009528.pub4. PMID: 25741986 Updated. Review.
-
Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis.Cochrane Database Syst Rev. 2012 Nov 14;11:CD009528. doi: 10.1002/14651858.CD009528.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Mar 05;(3):CD009528. doi: 10.1002/14651858.CD009528.pub3. PMID: 23152277 Updated. Review.
Cited by
-
"It Takes a Village": Mechanisms Underlying Antimicrobial Recalcitrance of Polymicrobial Biofilms.J Bacteriol. 2019 Dec 6;202(1):e00530-19. doi: 10.1128/JB.00530-19. Print 2019 Dec 6. J Bacteriol. 2019. PMID: 31548277 Free PMC article. Review.
-
Formation of a biofilm matrix network shapes polymicrobial interactions.ISME J. 2023 Mar;17(3):467-477. doi: 10.1038/s41396-023-01362-8. Epub 2023 Jan 13. ISME J. 2023. PMID: 36639539 Free PMC article.
-
Relationship of cell surface hydrophobicity with biofilm formation and growth rate: A study on Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli.Iran J Basic Med Sci. 2018 Jul;21(7):760-769. doi: 10.22038/IJBMS.2018.28525.6917. Iran J Basic Med Sci. 2018. PMID: 30140417 Free PMC article.
-
The Yin and Yang of Streptococcus Lung Infections in Cystic Fibrosis: a Model for Studying Polymicrobial Interactions.J Bacteriol. 2019 May 8;201(11):e00115-19. doi: 10.1128/JB.00115-19. Print 2019 Jun 1. J Bacteriol. 2019. PMID: 30885933 Free PMC article. Review.
-
Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance.Antibiotics (Basel). 2020 Dec 23;10(1):3. doi: 10.3390/antibiotics10010003. Antibiotics (Basel). 2020. PMID: 33374551 Free PMC article. Review.
References
-
- Fodor AA, Klem ER, Gilpin DF, Elborn JS, Boucher RC, Tunney MM, Wolfgang MC. 2012. The adult cystic fibrosis airway microbiota is stable over time and infection type, and highly resilient to antibiotic treatment of exacerbations. PLoS One 7:e45001. doi:10.1371/journal.pone.0045001. - DOI - PMC - PubMed
-
- Stressmann FA, Rogers GB, van der Gast CJ, Marsh P, Vermeer LS, Carroll MP, Hoffman L, Daniels TWV, Patel N, Forbes B, Bruce KD. 2012. Long-term cultivation-independent microbial diversity analysis demonstrates that bacterial communities infecting the adult cystic fibrosis lung show stability and resilience. Thorax 67:867–873. doi:10.1136/thoraxjnl-2011-200932. - DOI - PubMed
-
- Zhao J, Schloss PD, Kalikin LM, Carmody LA, Foster BK, Petrosino JF, Cavalcoli JD, VanDevanter DR, Murray S, Li JZ, Young VB, LiPuma JJ. 2012. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci U S A 109:5809–5814. doi:10.1073/pnas.1120577109. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

