Whole exome sequencing identifies causative mutations in the majority of consanguineous or familial cases with childhood-onset increased renal echogenicity
- PMID: 26489029
- PMCID: PMC4840095
- DOI: 10.1038/ki.2015.317
Whole exome sequencing identifies causative mutations in the majority of consanguineous or familial cases with childhood-onset increased renal echogenicity
Abstract
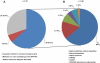
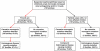
Chronically increased echogenicity on renal ultrasound is a sensitive early finding of chronic kidney disease that can be detected before manifestation of other symptoms. Increased echogenicity, however, is not specific for a certain etiology of chronic kidney disease. Here, we performed whole exome sequencing in 79 consanguineous or familial cases of suspected nephronophthisis in order to determine the underlying molecular disease cause. In 50 cases, there was a causative mutation in a known monogenic disease gene. In 32 of these cases whole exome sequencing confirmed the diagnosis of a nephronophthisis-related ciliopathy. In 8 cases it revealed the diagnosis of a renal tubulopathy. The remaining 10 cases were identified as Alport syndrome (4), autosomal-recessive polycystic kidney disease (2), congenital anomalies of the kidney and urinary tract (3), and APECED syndrome (1). In 5 families, in whom mutations in known monogenic genes were excluded, we applied homozygosity mapping for variant filtering and identified 5 novel candidate genes (RBM48, FAM186B, PIAS1, INCENP, and RCOR1) for renal ciliopathies. Thus, whole exome sequencing allows the detection of the causative mutation in 2/3 of affected individuals, thereby presenting the etiologic diagnosis, and allows identification of novel candidate genes.
Figures
Similar articles
-
Whole-exome resequencing distinguishes cystic kidney diseases from phenocopies in renal ciliopathies.Kidney Int. 2014 Apr;85(4):880-7. doi: 10.1038/ki.2013.450. Epub 2013 Nov 20. Kidney Int. 2014. PMID: 24257694 Free PMC article.
-
Targeted exome sequencing resolves allelic and the genetic heterogeneity in the genetic diagnosis of nephronophthisis-related ciliopathy.Exp Mol Med. 2016 Aug 5;48(8):e251. doi: 10.1038/emm.2016.63. Exp Mol Med. 2016. PMID: 27491411 Free PMC article.
-
Clinical and genetic analyses of a Dutch cohort of 40 patients with a nephronophthisis-related ciliopathy.Pediatr Nephrol. 2018 Oct;33(10):1701-1712. doi: 10.1007/s00467-018-3958-7. Epub 2018 Jul 5. Pediatr Nephrol. 2018. PMID: 29974258 Free PMC article.
-
Diagnosis of monogenic chronic kidney diseases.Curr Opin Nephrol Hypertens. 2019 Mar;28(2):183-194. doi: 10.1097/MNH.0000000000000486. Curr Opin Nephrol Hypertens. 2019. PMID: 30601180 Review.
-
Personalized medicine in chronic kidney disease by detection of monogenic mutations.Nephrol Dial Transplant. 2020 Mar 1;35(3):390-397. doi: 10.1093/ndt/gfz028. Nephrol Dial Transplant. 2020. PMID: 30809662 Free PMC article. Review.
Cited by
-
Mutations of ADAMTS9 Cause Nephronophthisis-Related Ciliopathy.Am J Hum Genet. 2019 Jan 3;104(1):45-54. doi: 10.1016/j.ajhg.2018.11.003. Am J Hum Genet. 2019. PMID: 30609407 Free PMC article.
-
Diagnostic Utility of Exome Sequencing for Kidney Disease.N Engl J Med. 2019 Jan 10;380(2):142-151. doi: 10.1056/NEJMoa1806891. Epub 2018 Dec 26. N Engl J Med. 2019. PMID: 30586318 Free PMC article.
-
Comprehensive Metabolic Signature of Renal Dysplasia in Children. A Multiplatform Metabolomics Concept.Front Mol Biosci. 2021 Jul 29;8:665661. doi: 10.3389/fmolb.2021.665661. eCollection 2021. Front Mol Biosci. 2021. PMID: 34395519 Free PMC article.
-
Review of genetic testing in kidney disease patients: Diagnostic yield of single nucleotide variants and copy number variations evaluated across and within kidney phenotype groups.Am J Med Genet C Semin Med Genet. 2022 Sep;190(3):358-376. doi: 10.1002/ajmg.c.31995. Epub 2022 Sep 26. Am J Med Genet C Semin Med Genet. 2022. PMID: 36161467 Free PMC article. Review.
-
Genomic medicine for kidney disease.Nat Rev Nephrol. 2018 Feb;14(2):83-104. doi: 10.1038/nrneph.2017.167. Epub 2018 Jan 8. Nat Rev Nephrol. 2018. PMID: 29307893 Free PMC article. Review.
References
-
- Kraus RA, Gaisie G, Young LW. Increased renal parenchymal echogenicity: causes in pediatric patients. Radiographics : a review publication of the Radiological Society of North America, Inc. 1990;10:1009–1018. - PubMed
-
- Moghazi S, Jones E, Schroepple J, et al. Correlation of renal histopathology with sonographic findings. Kidney international. 2005;67:1515–1520. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
- 5U54HG006504/HG/NHGRI NIH HHS/United States
- DK1068306/DK/NIDDK NIH HHS/United States
- UM1 HG006504/HG/NHGRI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- P30 DK079310/DK/NIDDK NIH HHS/United States
- K99 DK099434/DK/NIDDK NIH HHS/United States
- DK099434/DK/NIDDK NIH HHS/United States
- U54 HG006504/HG/NHGRI NIH HHS/United States
- R01 DK068306/DK/NIDDK NIH HHS/United States
- R00 DK099434/DK/NIDDK NIH HHS/United States
- DK064614/DK/NIDDK NIH HHS/United States
- R01 DK064614/DK/NIDDK NIH HHS/United States
- DK1069274/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

