AMSH interacts with ESCRT-0 to regulate the stability and trafficking of CXCR4
- PMID: 20159979
- PMCID: PMC2859561
- DOI: 10.1074/jbc.M109.061309
AMSH interacts with ESCRT-0 to regulate the stability and trafficking of CXCR4
Abstract
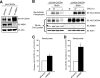
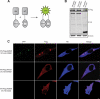
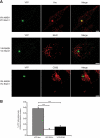
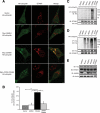
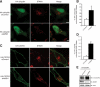
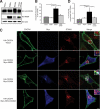
Reversible ubiquitination is essential for the endocytic sorting and down-regulation of G protein-coupled receptors, such as the chemokine receptor CXCR4. The deubiquitinating enzyme AMSH has been implicated in the endocytic sorting of both G protein-coupled receptors and receptor-tyrosine kinases. Herein, we examine the role of AMSH in the regulation of CXCR4 stability and trafficking and characterize protein-protein interactions critical for this function. Loss of AMSH catalytic activity or depletion by RNAi results in increased steady-state levels of CXCR4 under basal conditions. Analysis of truncation and point mutation of AMSH reveal the importance of an RXXK motif for CXCR4 degradation. The RXXK motif of AMSH interacts with the SH3 domains of the STAM and Grb2 families of adaptor proteins with high affinity. Cells expressing a catalytically inactive mutant of AMSH show basal hyperubiquitination, but not increased degradation, of the ESCRT-0 components STAM1 and Hrs. This is dependent on the RXXK motif of AMSH. Ubiquitination of endocytic machinery modulates their activity, suggesting that AMSH may directly regulate endocytic adaptor protein function. This is reflected in CXCR4 trafficking and provides a mechanism by which AMSH specifies the fate of endocytosed receptors. Taken together, these studies implicate AMSH as a key modulator of receptor fate determination through its action on components of the endocytic machinery.
Figures


Similar articles
-
The Vps27/Hrs/STAM (VHS) Domain of the Signal-transducing Adaptor Molecule (STAM) Directs Associated Molecule with the SH3 Domain of STAM (AMSH) Specificity to Longer Ubiquitin Chains and Dictates the Position of Cleavage.J Biol Chem. 2016 Jan 22;291(4):2033-2042. doi: 10.1074/jbc.M115.689869. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601948 Free PMC article.
-
Regulation of epidermal growth factor receptor ubiquitination and trafficking by the USP8·STAM complex.J Biol Chem. 2010 Nov 5;285(45):34909-21. doi: 10.1074/jbc.M109.016287. Epub 2010 Aug 24. J Biol Chem. 2010. PMID: 20736164 Free PMC article.
-
The deubiquitinating enzyme USP8 promotes trafficking and degradation of the chemokine receptor 4 at the sorting endosome.J Biol Chem. 2010 Nov 26;285(48):37895-908. doi: 10.1074/jbc.M110.129411. Epub 2010 Sep 27. J Biol Chem. 2010. PMID: 20876529 Free PMC article.
-
Regulation of endocytic sorting by ESCRT-DUB-mediated deubiquitination.Cell Biochem Biophys. 2011 Jun;60(1-2):39-46. doi: 10.1007/s12013-011-9181-9. Cell Biochem Biophys. 2011. PMID: 21448666 Review.
-
The Hrs/STAM complex in the downregulation of receptor tyrosine kinases.J Biochem. 2005 Jan;137(1):1-8. doi: 10.1093/jb/mvi001. J Biochem. 2005. PMID: 15713877 Review.
Cited by
-
Mutations in STAMBP, encoding a deubiquitinating enzyme, cause microcephaly-capillary malformation syndrome.Nat Genet. 2013 May;45(5):556-62. doi: 10.1038/ng.2602. Epub 2013 Mar 31. Nat Genet. 2013. PMID: 23542699 Free PMC article.
-
Arrestin-2 interacts with the endosomal sorting complex required for transport machinery to modulate endosomal sorting of CXCR4.Mol Biol Cell. 2010 Jul 15;21(14):2529-41. doi: 10.1091/mbc.e10-02-0169. Epub 2010 May 26. Mol Biol Cell. 2010. PMID: 20505072 Free PMC article.
-
Monoubiquitination of EEA1 regulates endosome fusion and trafficking.Cell Biosci. 2013 May 23;3(1):24. doi: 10.1186/2045-3701-3-24. Cell Biosci. 2013. PMID: 23701900 Free PMC article.
-
Ubiquitin-like protein conjugation and the ubiquitin-proteasome system as drug targets.Nat Rev Drug Discov. 2011 Jan;10(1):29-46. doi: 10.1038/nrd3321. Epub 2010 Dec 10. Nat Rev Drug Discov. 2011. PMID: 21151032 Free PMC article. Review.
-
The Vps27/Hrs/STAM (VHS) Domain of the Signal-transducing Adaptor Molecule (STAM) Directs Associated Molecule with the SH3 Domain of STAM (AMSH) Specificity to Longer Ubiquitin Chains and Dictates the Position of Cleavage.J Biol Chem. 2016 Jan 22;291(4):2033-2042. doi: 10.1074/jbc.M115.689869. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601948 Free PMC article.
References
-
- von Zastrow M. (2003) Life Sci 74, 217–224 - PubMed
-
- Sorkin A., Von Zastrow M. (2002) Nat. Rev. Mol. Cell Biol. 3, 600–614 - PubMed
-
- Lefkowitz R. J. (1998) J. Biol. Chem. 273, 18677–18680 - PubMed
-
- Tsao P., Cao T., von Zastrow M. (2001) Trends Pharmacol Sci 22, 91–96 - PubMed
-
- Di Fiore P. P., Polo S., Hofmann K. (2003) Nat. Rev. Mol. Cell Biol. 4, 491–497 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

