Molecular basis of reduced pyridoxine 5'-phosphate oxidase catalytic activity in neonatal epileptic encephalopathy disorder
- PMID: 19759001
- PMCID: PMC2781495
- DOI: 10.1074/jbc.M109.038372
Molecular basis of reduced pyridoxine 5'-phosphate oxidase catalytic activity in neonatal epileptic encephalopathy disorder
Abstract
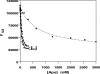
Mutations in pyridoxine 5'-phosphate oxidase are known to cause neonatal epileptic encephalopathy. This disorder has no cure or effective treatment and is often fatal. Pyridoxine 5'-phosphate oxidase catalyzes the oxidation of pyridoxine 5'-phosphate to pyridoxal 5'-phosphate, the active cofactor form of vitamin B(6) required by more than 140 different catalytic activities, including enzymes involved in amino acid metabolism and biosynthesis of neurotransmitters. Our aim is to elucidate the mechanism by which a homozygous missense mutation (R229W) in the oxidase, linked to neonatal epileptic encephalopathy, leads to reduced oxidase activity. The R229W variant is approximately 850-fold less efficient than the wild-type enzyme due to an approximately 192-fold decrease in pyridoxine 5'-phosphate affinity and an approximately 4.5-fold decrease in catalytic activity. There is also an approximately 50-fold reduction in the affinity of the R229W variant for the FMN cofactor. A 2.5 A crystal structure of the R229W variant shows that the substitution of Arg-229 at the FMN binding site has led to a loss of hydrogen-bond and/or salt-bridge interactions between FMN and Arg-229 and Ser-175. Additionally, the mutation has led to an alteration of the configuration of a beta-strand-loop-beta-strand structure at the active site, resulting in loss of two critical hydrogen-bond interactions involving residues His-227 and Arg-225, which are important for substrate binding and orientation for catalysis. These results provide a molecular basis for the phenotype associated with the R229W mutation, as well as providing a foundation for understanding the pathophysiological consequences of pyridoxine 5'-phosphate oxidase mutations.
Figures

Similar articles
-
Active site structure and stereospecificity of Escherichia coli pyridoxine-5'-phosphate oxidase.J Mol Biol. 2002 Jan 18;315(3):385-97. doi: 10.1006/jmbi.2001.5254. J Mol Biol. 2002. PMID: 11786019
-
X-ray structure of Escherichia coli pyridoxine 5'-phosphate oxidase complexed with pyridoxal 5'-phosphate at 2.0 A resolution.J Mol Biol. 2001 Jul 20;310(4):817-26. doi: 10.1006/jmbi.2001.4734. J Mol Biol. 2001. PMID: 11453690
-
Structures of Mycobacterium tuberculosispyridoxine 5'-phosphate oxidase and its complexes with flavin mononucleotide and pyridoxal 5'-phosphate.Acta Crystallogr D Biol Crystallogr. 2005 Nov;61(Pt 11):1492-9. doi: 10.1107/S0907444905026673. Epub 2005 Oct 19. Acta Crystallogr D Biol Crystallogr. 2005. PMID: 16239726
-
Structure and mechanism of Escherichia coli pyridoxine 5'-phosphate oxidase.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):76-82. doi: 10.1016/s1570-9639(03)00060-8. Biochim Biophys Acta. 2003. PMID: 12686112 Review.
-
Inborn errors in the vitamin B6 salvage enzymes associated with neonatal epileptic encephalopathy and other pathologies.Biochimie. 2021 Apr;183:18-29. doi: 10.1016/j.biochi.2020.12.025. Epub 2021 Jan 6. Biochimie. 2021. PMID: 33421502 Free PMC article. Review.
Cited by
-
Allosteric feedback inhibition of pyridoxine 5'-phosphate oxidase from Escherichia coli.J Biol Chem. 2019 Oct 25;294(43):15593-15603. doi: 10.1074/jbc.RA119.009697. Epub 2019 Sep 4. J Biol Chem. 2019. PMID: 31484724 Free PMC article.
-
Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5.Nutrients. 2022 Jan 22;14(3):484. doi: 10.3390/nu14030484. Nutrients. 2022. PMID: 35276844 Free PMC article. Review.
-
Pyridoxine responsiveness in novel mutations of the PNPO gene.Neurology. 2014 Apr 22;82(16):1425-33. doi: 10.1212/WNL.0000000000000344. Epub 2014 Mar 21. Neurology. 2014. PMID: 24658933 Free PMC article.
-
Improving Treatment Options for Primary Hyperoxaluria.Drugs. 2022 Jul;82(10):1077-1094. doi: 10.1007/s40265-022-01735-x. Epub 2022 Jul 2. Drugs. 2022. PMID: 35779234 Free PMC article. Review.
-
Proteomic profiling reveals that resveratrol inhibits HSP27 expression and sensitizes breast cancer cells to doxorubicin therapy.PLoS One. 2013 May 27;8(5):e64378. doi: 10.1371/journal.pone.0064378. Print 2013. PLoS One. 2013. PMID: 23724044 Free PMC article.
References
-
- Mills P. B., Surtees R. A., Champion M. P., Beesley C. E., Dalton N., Scambler P. J., Heales S. J., Briddon A., Scheimberg I., Hoffmann G. F., Zschocke J., Clayton P. T. (2005) Hum. Mol. Genet. 14, 1077–1086 - PubMed
-
- Clayton P. T., Surtees R. A., DeVile C., Hyland K., Heales S. J. (2003) Lancet 361, 1614–1614 - PubMed
-
- John R. A. (1995) Biochim. Biophys. Acta 1248, 81–96 - PubMed
-
- Leklem J. E. (1991) in Handbook of Vitamins (Machlin L. ed) pp. 341–378, Marcel Dekker, Inc., New York
-
- McCormick D. B. (1996) in Encyclopedia of Molecular Biology and Molecular Medicine (Meyers R. A. ed) pp. 396–406, Wiley-VCH, Weinheim, Germany
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

