Neuro-ophthalmologic and electroretinographic findings in pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome)
- PMID: 16023068
- PMCID: PMC2169522
- DOI: 10.1016/j.ajo.2005.03.024
Neuro-ophthalmologic and electroretinographic findings in pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome)
Abstract
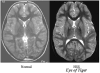
Purpose: The onset of pantothenate kinase-associated neurodegeneration (PKAN) occurs in the first and second decade of life and a pigmentary retinal degeneration is a feature of the disorder. Since the neuro-ophthalmologic and electroretinographic (ERG) features have never been well delineated, we describe them in 16 patients with PKAN.
Design: Observational case series.
Methods: Sixteen patients with genetic and neuroimaging-confirmed PKAN were examined. Ten underwent neuro-ophthalmologic examination and all had ERGs.
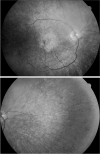
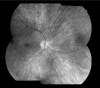
Results: Of the 10 who underwent neuro-ophthalmologic examination, all showed saccadic pursuits and eight showed hypometric or slowed vertical saccades. Seven of eight had inability to suppress the vestibulo-ocular reflex; two patients could not cooperate. Two had square wave jerks and four had poor convergence. Vertical optokinetic responses were abnormal in five, and two patients had blepharospasm. Eight patients had sectoral iris paralysis and partial loss of the pupillary ruff consistent with Adie's pupils in both eyes. Only four of 10 examined patients showed a pigmentary retinopathy, but 11 of 16 had abnormal ERGs ranging from mild cone abnormalities to severe rod-cone dysfunction. No patient had optic atrophy. The PANK2 mutations of all of the patients were heterogeneous.
Conclusions: Adie's-like pupils, abnormal vertical saccades, and saccadic pursuits were very common. These findings suggest that mid-brain degeneration occurs in PKAN more frequently than previously thought. ERG abnormalities were present in approximately 70% and no patient had optic atrophy. Although genotype-ocular phenotype correlations could not be established, allelic differences probably contributed to the variable clinical expression of retinopathy and other clinical characteristics in these patients.
Figures
Similar articles
-
Clinical heterogeneity of neurodegeneration with brain iron accumulation (Hallervorden-Spatz syndrome) and pantothenate kinase-associated neurodegeneration.Mov Disord. 2004 Jan;19(1):36-42. doi: 10.1002/mds.10650. Mov Disord. 2004. PMID: 14743358
-
Siblings with the adult-onset slowly progressive type of pantothenate kinase-associated neurodegeneration and a novel mutation, Ile346Ser, in PANK2: clinical features and (99m)Tc-ECD brain perfusion SPECT findings.J Neurol Sci. 2010 Mar 15;290(1-2):172-6. doi: 10.1016/j.jns.2009.11.008. Epub 2009 Dec 14. J Neurol Sci. 2010. PMID: 20006850
-
Unraveling the Hallervorden-Spatz syndrome: pantothenate kinase-associated neurodegeneration is the name.Curr Opin Pediatr. 2003 Dec;15(6):572-7. doi: 10.1097/00008480-200312000-00005. Curr Opin Pediatr. 2003. PMID: 14631201 Review.
-
Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome.N Engl J Med. 2003 Jan 2;348(1):33-40. doi: 10.1056/NEJMoa020817. N Engl J Med. 2003. PMID: 12510040
-
Pantothenate kinase-associated neurodegeneration (Hallervorden-Spatz syndrome).Eur J Paediatr Neurol. 2002;6(5):243-7. doi: 10.1053/ejpn.2002.0606. Eur J Paediatr Neurol. 2002. PMID: 12374576 Review.
Cited by
-
Neurodegeneration with brain iron accumulation: an overview.Iran J Child Neurol. 2014 Fall;8(4):1-8. Iran J Child Neurol. 2014. PMID: 25657764 Free PMC article. Review.
-
Microarray analysis of murine retinal light damage reveals changes in iron regulatory, complement, and antioxidant genes in the neurosensory retina and isolated RPE.Invest Ophthalmol Vis Sci. 2012 Aug 7;53(9):5231-41. doi: 10.1167/iovs.12-10204. Invest Ophthalmol Vis Sci. 2012. PMID: 22736611 Free PMC article.
-
Pantothenate kinase-associated neurodegeneration: Clinical aspects, diagnosis and treatments.Neurol Int. 2018 Apr 4;10(1):7516. doi: 10.4081/ni.2018.7516. eCollection 2018 Mar 30. Neurol Int. 2018. PMID: 29844889 Free PMC article.
-
β-Propeller protein-associated neurodegeneration: a new X-linked dominant disorder with brain iron accumulation.Brain. 2013 Jun;136(Pt 6):1708-17. doi: 10.1093/brain/awt095. Epub 2013 May 17. Brain. 2013. PMID: 23687123 Free PMC article.
-
Neurodegeneration with Brain Iron Accumulation.Curr Neurol Neurosci Rep. 2016 Jan;16(1):9. doi: 10.1007/s11910-015-0608-3. Curr Neurol Neurosci Rep. 2016. PMID: 26739693 Review.
References
-
- Swaiman KF. Hallervorden-Spatz syndrome and brain iron metabolism. Arch Neurol. 1991;48:1285–1293. - PubMed
-
- Dooling EC, Schoene WC, Richardson EP., Jr Hallervorden-Spatz syndrome. Arch Neurol. 1974;30:70–83. - PubMed
-
- Thomas M, Hayflick SJ, Jankovic J. Clinical heterogeneity of neurodegeneration with brain iron accumulation (Hallervorden-Spatz syndrome) and pantothenate kinase-associated neurodegeneration. Mov Disord. 2004;19:36–42. - PubMed
-
- Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet. 2001;28:345–349. - PubMed
-
- Neumann M, Adler S, Schluter O, Kremmer E, Benecke R, Kretzschmar HA. Alpha-synuclein accumulation in a case of neurodegeneration with brain iron accumulation type 1(NBIA-1, formerly Hallervorden-Spatz syndrome) with widespread cortical and brainstem-type Lewy bodies. ActaNeuropathol (Berl) 2000;100:568–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

