NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Vismodegib is an orally available, small molecule inhibitor of the Hedgehog pathway that is used to treat unresectable or metastatic basal cell carcinoma. Vismodegib therapy is associated with a low rate or transient elevations in serum aminotransferase during therapy and has been linked to rare cases of clinically apparent acute liver injury that can be severe and even fatal.
Background
Vismodegib (vis” moe deg’ ib) is an orally available, small molecule inhibitor of a key step in the hedgehog signaling pathway (activation of smoothened: SMO). Hedgehog is an important regulator of embryonic development, cell growth and differentiation. Mutations in this pathway have been identified in several malignant diseases including basal cell carcinoma. Clinical trials of vismodegib in patients with metastatic or locally advanced basal cell carcinoma reported at least partial responses in up to half of patients. Vismodegib, the first hedgehog pathway inhibitor, was approved for use in the United States in 2012; sonidegib the second such inhibitor, was approved in 2015. Current indications include metastatic or locally advanced, recurrent, or unresectable basal cell carcinoma. Vismodegib is available in capsules of 150 mg under the brand name Erivedge. The typical dose is 150 mg once daily until disease progression or unacceptable toxicity occurs. Side effects are common and often dose limiting, although rarely life threatening. Common side effects include muscle spasms, alopecia, anorexia, dysgeusia, weight loss, nausea, diarrhea, fatigue and arthralgias. Potential serious adverse events include severe weight loss, squamous cell skin cancer, and embryo-fetal toxicity. Vismodegib has a black box warning of fetal and embryonal toxicity with its use and advises screening for pregnancy before starting therapy and use of effective birth control during and for 20 months following treatment.
Hepatotoxicity
Most clinical trials of vismodegib included few patients, and rates of liver tests abnormalities were usually not reported. The product label for vismodegib includes no mention serum enzyme elevations or need for monitoring of routine liver tests. In a subsequent review of all published studies of vismodegib, liver enzyme elevations were said to occur in 1.4% of a total of 363 patients treated. Since its approval and more general use, reports of clinically apparent liver injury linked to vismodegib have appeared, and the product label now mentions adverse events of hepatotoxicity and Stevens Johnson syndrome in post marketing reports. In one published report, an elderly man presented with fatigue, nausea, and jaundice 41 days after starting vismodegib with a cholestatic pattern of serum enzyme elevations and rapid improvement on stopping (Case 1). In addition, review of 7 years of spontaneous adverse event reporting to the FDA revealed 94 reports of hepatotoxicity during vismodegib therapy, including 20 that were considered serious and 4 that resulted in hepatic failure. The latency to onset was typically within 2 to 12 weeks of starting vismodegib and the pattern of injury was typically cholestatic, although less commonly hepatocellular. Autoimmune features are not common but in several reports there were features of immune allergic reactions (rash, eosinophilia, fever). Thus, clinically apparent liver injury from vismodegib occurs, but is somewhat rare.
Likelihood score: B (likely rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of liver injury from vismodegib is unknown, but likely due to hypersensitivity. Vismodegib has a prolonged half-life (~19 days) and is metabolized at least in part in the liver via multiple cytochrome P450 enzymes, including CYP 3A4, 2C8, 2C9 and 2C19. While it theoretically should have several drug-drug interactions, there is little clinical data on its effects on other drugs or vice versa.
Outcome and Management
In using kinase and small molecule inhibitors for treatment of cancer, monitoring of routine liver tests before starting and at intervals during therapy is warranted. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) or any elevations accompanied by jaundice or symptoms should lead to at least temporary cessation. Restarting vismodegib after cessation for liver test abnormalities should be done with caution, and only after the abnormalities have resolved or improved significantly, and in patients who exhibit a clinical response to the agent. In some cases, use of a lower dose provided continued clinical efficacy without recurrence of the liver test abnormalities. The various protein kinase inhibitors vary greatly in chemical structure and there is little evidence for cross sensitivity to the liver injury.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
Other Drugs for Basal Cell Carcinoma: Sonidegib
CASE REPORT
Case 1. Cholestatic hepatitis attributed to vismodegib therapy.(1)
A 72 year old man with multiple basal cell carcinomas developed worsening nausea, dysgeusia, anorexia, weight loss and muscle cramps followed by appearance of jaundice 5 to 6 weeks after starting vismodegib. He also had a sudden loss of voice, decreased fluid intake and melena without sore throat, fever or rash. He had no history of liver disease or alcohol abuse, and all liver tests had been normal before vismodegib therapy was begun (Table). Medications that he took chronically included tadalafil, amlodipine, and valsartan, and he had recently started naproxen and aspirin because of muscle cramps attributed to vismodegib. On presentation, he was jaundiced and had mild hepatic tenderness. Serum bilirubin was 10.7 mg/dL, ALT 525 U/L, AST 206 U/L, alkaline phosphatase 807 U/L, GGT 550 U/L and INR 1.1. He had a mild increase in serum creatinine (2.2 mg/dL), possibly due to dehydration. Vismodegib was stopped and he was admitted for evaluation and intravenous hydration. Imaging of the liver showed no evidence of gall stones or biliary obstruction. Symptoms and liver test abnormalities began to improve rapidly and two weeks later, jaundice had resolved and liver tests were minimally elevated. Six months later, routine liver tests were all within the normal range.
Key Points
| Medication: | Vismodegib (300 mg daily) |
|---|---|
| Pattern: | Cholestatic (R=1.2) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 5-6 weeks |
| Recovery: | ~4 weeks |
| Other medications: | Aspirin and naproxen acutely; tadalafil, amlodipine and valsartan chronically. |
Laboratory Values
Comment
An elderly man with multiple basal cell carcinomas developed dysgeusia and muscle cramps within a month of starting vismodegib and was found to have mild serum ALT and AST elevations. In the following two weeks, however, he developed worsening symptoms and jaundice and was found to have a cholestatic hepatitis. He had also developed worsening side effects of treatment, dehydration, laryngitis and mild gastrointestinal bleeding. Upon hospital admission and stopping vismodegib he improved rapidly, most liver tests being normal or near normal two weeks later. Information on hepatitis serology, autoantibodies and eosinophil counts was not provided, but immunoallergic features were not present, and the clinical course and outcome were entirely compatible with a drug induced cholestatic hepatitis. While most cases of acute liver injury attributed to kinase inhibitors have been described as hepatocellular, the actual aminotransferase and alkaline phosphatase values are often not provided. Even this case might have been considered hepatocellular (ALT 525 U/L) had the other values not been available.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Vismodegib – Erivedge®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Vismodegib | 879085-55-9 | C19-H14-Cl2-N2-O3-S |
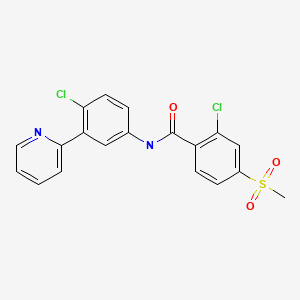
|
CITED REFERENCES
- 1.
- Ash MM, Jolly PS. Cholestatic hepatic injury associated with vismodegib, aspirin, and naproxen use: a case study and review of vismodegib safety. Int J Dermatol 2015; 54: 370-4. [PubMed: 25039741]
ANNOTATED BIBLIOGRAPHY
References updated: 06 January 2025
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors such as vismodegib).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents discusses several tyrosine kinase inhibitors including imatinib, gefitinib, erlotinib and crizotinib, but not vismodegib).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, Weiss GJ, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med 2009; 361: 1164-72. [PubMed: 19726763](Among 33 patients with metastatic or locally advanced basal cell carcinoma treated with vismodegib in varying doses, 18 [55%] had an objective response and adverse events included fatigue, hyponatremia, weight loss, dyspnea and prolonged QTc interval; no mention of ALT elevations, but one patient had an Alk P elevation ≥3 times ULN).
- LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res 2011; 17: 2502-11. [PMC free article: PMC5244484] [PubMed: 21300762](Among 68 patients with locally advanced or metastatic solid tumors treated with vismodegib, tumor responses occurred in 20 patients [29%], all but one with basal cell carcinoma; difficult side effects included hyponatremia, abdominal pain and fatigue; no mention of liver injury).
- Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med 2012; 366: 2171-9. [PMC free article: PMC5278761] [PubMed: 22670903](Among 96 patients with advanced or metastatic basal cell cancer treated with vismodegib, the response rate was 40% and side effects were common including muscle spasms, alopecia, dysgeusia, weight loss, fatigue, nausea and diarrhea, and were considered severe in 26% of patients; no mention of ALT elevations or hepatotoxcity).
- Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, et al. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med 2012; 366: 2180-8. [PMC free article: PMC4362529] [PubMed: 22670904](Among 41 patients with basal cell nevus syndrome, vismodegib therapy was associated with a lower rate of new tumors and decrease in size of existing tumors compared to placebo, but was associated with a high rate of side effects and 54% stopped therapy because of side effects; no mention of ALT elevations or hepatotoxicity).
- Vismodegib (Erivedge) for basal cell carcinoma. Med Lett Drugs Ther 2012; 54: 53-4. [PubMed: 22777303](Concise summary of mechanism of action, efficacy, safety, and cost of vismodegib for basal cell carcinoma shortly after its approval in the US mentions that side effects are common, but not life-threatening).
- Spraggs CF, Xu CF, Hunt CM. Genetic characterization to improve interpretation and clinical management of hepatotoxicity caused by tyrosine kinase inhibitors. Pharmacogenomics 2013; 14: 541-54. [PubMed: 23556451](Review of genetic associations of serum ALT and bilirubin elevations during therapy with tyrosine kinase inhibitors, focusing on lapatinib and pazopanib).
- Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf 2013; 36: 491-503. [PubMed: 23620168](Review of the hepatotoxicity of 18 tyrosine kinase inhibitors approved for use in cancer in the US as of 2013; vismodegib is not discussed).
- Ash MM, Jolly PS. Cholestatic hepatic injury associated with vismodegib, aspirin, and naproxen use: a case study and review of vismodegib safety. Int J Dermatol. 2015;54:370-4. [PubMed: 25039741](72 year old man with history of multiple basal cell carcinomas developed nausea and jaundice 4 to 6 weeks after starting vismodegib [bilirubin 10.7 mg/dL, ALT 525 U/L, Alk P 807 U/L], symptoms and jaundice resolving within 2 weeks of stopping and all tests were normal 6 months later: Case 1).
- Ventarola DJ, Silverstein DI. Vismodegib-associated hepatotoxicity: a potential side effect detected in postmarketing surveillance. J Am Acad Dermatol. 2014;71:397-8. [PubMed: 25037792](Within a year of approval in the US, 65 patients with adverse reactions had been reported to the FDA, including 15 with liver toxicity; no clinical details provided).
- Edwards BJ, Raisch DW, Saraykar SS, Sun M, Hammel JA, Tran HT, Wehr N, et al. Hepatotoxicity with vismodegib: an MD Anderson Cancer Center and Research on Adverse Drug Events and Reports Project. Drugs R D. 2017;17:211-218. [PMC free article: PMC5318336] [PubMed: 28063021](Search of the FDAs Adverse Event Reporting System from 2009 to 2015 identified 35 reports of severe liver injury attributed to vismodegib, 20 of which were scored as serious and 4 as causing acute liver failure and death, all reported between 2011 and 2015).
- Sekulic A, Migden MR, Basset-Seguin N, Garbe C, Gesierich A, Lao CD, Miller C, et al.; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332. [PMC free article: PMC5433030] [PubMed: 28511673](In a long term follow up [39 months] after enrollment of 104 patients into a trial of vismodegib for metastatic or locally advanced basal cell carcinoma [Sekulic 2012], responses were sustained, and no new adverse events were identified but only 8 patients remained on therapy; no mention of ALT elevations or clinically apparent liver injury).
- Dréno B, Kunstfeld R, Hauschild A, Fosko S, Zloty D, Labeille B, Grob JJ, et al. Two intermittent vismodegib dosing regimens in patients with multiple basal-cell carcinomas (MIKIE): a randomised, regimen-controlled, double-blind, phase 2 trial. Lancet Oncol. 2017;18:404-412. [PubMed: 28188086](Among 229 patients with basal cell carcinoma treated with two regimens of vismodegib, adverse events included muscle spasms [77 and 83%], alopecia [63 and 65%], fatigue [21 and 23%], weight loss [19 and 21%], nausea [13 and 20%] and GGT elevations [2 and 4%]; no mention of ALT elevations, discontinuations for liver enzyme elevations, or clinically apparent liver injury).
- Bedi PS, Rai MP, Tageja N, Laird-Fick H. Hepatotoxicity associated with vismodegib. BMJ Case Rep. 2018;2018:bcr2017222969. [PMC free article: PMC5836615] [PubMed: 29437771](82 year old woman with basal cell carcinomas developed nausea, abdominal pain, and jaundice 2 months after starting vismodegib [bilirubin 17 rising to 29 mg/dL, ALT 204 U/L, Alk P 232 U/L, INR 3.2], with slow recovery after stopping).
- Ly P, Wolf K, Wilson J. A case of hepatotoxicity associated with vismodegib. JAAD Case Rep. 2018;5:57-59. [PMC free article: PMC6289949] [PubMed: 30560186](54 year old man with locally advanced basal cell carcinoma developed pruritus and jaundice 5 weeks after starting vismodegib [bilirubin 6.3 mg/dL, ALT 151 U/L, Alk P 304 U/L], resolving after stopping the drug).
- Drago F, Trave I, Wei Y, Parodi A. Skin eruption and cholestatic hepatic injury due to vismodegib. G Ital Dermatol Venereol. 2019;154:496-497. [PubMed: 29199802](69 year old woman with large, refractory basal cell carcinoma developed abdominal pain, skin rash, and jaundice 2 months after starting vismodegib [bilirubin 12.7 mg/dL, ALT 226 U/L, Alk P 665 U/L, eosinophilia 9.5%], resolving rapidly after stopping drug).
- de Perosanz-Lobo D, Burgos-Blasco P, Moreno-Arrones OM, Bea-Ardebol S. Hepatotoxicity associated with vismodegib: could dose reduction be an effective management? Dermatol Surg. 2021;47:1006-1007. [PubMed: 34167133](67 year old woman with locally advanced basal cell carcinoma was found to have elevations in serum ALT [4 times ULN, bilirubin and Alk P not provided] 3 months after starting vismodegib, which resolved rapidly on stopping and did not recur when she was restarted on vismodegib at a lower dose [150 mg every other day]).
- Sekulic A, Yoo S, Kudchadkar R, Guillen J, Rogers G, Chang ALS, Guenthner S, et al. Real-world assessment and treatment of locally advanced basal cell carcinoma: Findings from the RegiSONIC disease registry. PLoS One. 2022;17:e0262151. [PMC free article: PMC8759646] [PubMed: 35030185](Among 433 patients with locally advanced basal cell carcinoma enrolled in a prospective registry, 115 received vismodegib among whom the most common adverse events were dysgeusia, muscle spasms, alopecia, and weight loss; no mention of ALT elevations or clinically apparent liver injury).
- Gutzmer R, Loquai C, Robert C, Dréno B, Guminski A, Lewis K, Arntz R, et al. Key clinical adverse events in patients with advanced basal cell carcinoma treated with sonidegib or vismodegib: a post hoc analysis. Dermatol Ther (Heidelb). 2021;11:1839-1849. [PMC free article: PMC8484385] [PubMed: 34490549](Among patients with locally advanced or metastatic basal cell carcinoma treated in separate studies with sonidegib [200 mg daily: n=79] or vismodegib [150 mg daily: n=119], common side effects included muscle spasms [54 vs 71%], alopecia [49 vs 58%], dysgeusia [43 vs 71%], diarrhea [32 vs 25%], fatigue [39 vs 19%], and weight loss [33 vs 19%]; no mention of ALT elevations or clinically apparent liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Variegate Porphyria.[GeneReviews(®). 1993]Review Variegate Porphyria.Singal AK, Anderson KE. GeneReviews(®). 1993
- Genetics, X-Linked Inheritance.[StatPearls. 2025]Genetics, X-Linked Inheritance.Basta M, Pandya AM. StatPearls. 2025 Jan
- Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.[JBJS Essent Surg Tech. 2024]Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.Manes TJ, DeGenova DT, Taylor BC, Patel JN. JBJS Essent Surg Tech. 2024 Oct-Dec; 14(4). Epub 2024 Dec 6.
- Review FBN1-Related Marfan Syndrome.[GeneReviews(®). 1993]Review FBN1-Related Marfan Syndrome.Dietz H. GeneReviews(®). 1993
- Immediate Hypersensitivity Reactions (Archived).[StatPearls. 2025]Immediate Hypersensitivity Reactions (Archived).Justiz Vaillant AA, Vashisht R, Zito PM. StatPearls. 2025 Jan
- Vismodegib - LiverToxVismodegib - LiverTox
- Vinca Alkaloids - LiverToxVinca Alkaloids - LiverTox
- Viltolarsen - LiverToxViltolarsen - LiverTox
- Viloxazine - LiverToxViloxazine - LiverTox
- Vilazodone - LiverToxVilazodone - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...