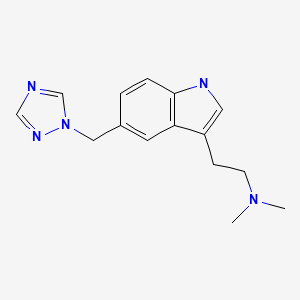
CASRN: 144034-80-0
Drug Levels and Effects
Summary of Use during Lactation
Breastmilk levels of rizatriptan are low and the half-life in milk is relatively short. Amounts ingested by the infant are small and unlikely to affect the nursing infant. Painful, burning nipples and breast pain have been reported after doses of sumatriptan and other triptans. This has occasionally been accompanied by a decrease in milk production.
Drug Levels
Maternal Levels. Five women who were at least 1 month postpartum and used rizatriptan to treat migraine provided one milk sample before their dose, then additional milk samples at 1, 2, 4, 8, 12 and 24 hours after their 10 mg oral dose. The average peak milk level was 58.4 mcg/L (range 14.6 to 105.6 mcg/L) and occurred 2 hours after the dose in 4 women ad 4 hours after the dose in another. The average milk level was 9 mcg/L (range 2.6 to 14.8 mcg/L) and the average half-life in milk was 2.2 hours (range 1.6 to 3.1 hours). The average daily infant dosage of rizatriptan was 1.3 mcg/kg (range 0.4 to 2.2 mcg/kg) and the weight-adjusted infant dosage averaged 0.9% (range 0.5 to 1.4%) of the maternal dose.[1]
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
A review of four European adverse reaction databases found 26 reported cases of, painful, burning nipples, painful breasts, breast engorgement and/or painful milk ejection in women who took a triptan while nursing. Pain was sometimes intense and occasionally led to decreased milk production. Pain generally subsided with time as the drug was eliminated. The authors proposed that triptans may cause vasoconstriction of the arteries in the breast, nipples, and the arteries surrounding the alveoli and milk ducts, causing a painful sensation and a painful milk ejection reflex.[2]
Alternate Drugs to Consider
References
- 1.
- Amundsen S, Nordeng H, Fuskevåg OM, et al. Transfer of triptans into human breast milk and estimation of infant drug exposure through breastfeeding. Basic Clin Pharmacol Toxicol 2021;128:795-804. [PubMed: 33730376]
- 2.
- Conijn M, Maas V, van Tuyl M, et al. Breastfeeding-related adverse drug reactions of triptans: A descriptive analysis using four pharmacovigilance databases. Breastfeed Med 2024;19:645-51. [PubMed: 38563407]
Substance Identification
Substance Name
Rizatriptan
CAS Registry Number
144034-80-0
Drug Class
Breast Feeding
Lactation
Milk, Human
Serotonin Receptor Agonists
Serotonin 5-HT1 Receptor Agonists
Triptans
Vasoconstrictor Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
Publication Details
Publication History
Last Revision: September 15, 2024.
Copyright
Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
Publisher
National Institute of Child Health and Human Development, Bethesda (MD)
NLM Citation
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. Rizatriptan. [Updated 2024 Sep 15].