Weekly Somapacitan is Effective and Well Tolerated in Children With GH Deficiency: The Randomized Phase 3 REAL4 Trial
- PMID: 36062966
- PMCID: PMC9693810
- DOI: 10.1210/clinem/dgac513
Weekly Somapacitan is Effective and Well Tolerated in Children With GH Deficiency: The Randomized Phase 3 REAL4 Trial
Abstract
Context: Somapacitan, a once-weekly reversible albumin-binding GH derivative, is evaluated in children with GH deficiency (GHD).
Objective: To demonstrate efficacy and safety of somapacitan vs daily GH.
Methods: REAL4 is a randomised, multinational, open-labeled, active-controlled parallel group phase 3 trial, comprising a 52-week main trial and 3-year extension (NCT03811535).
Setting: Eighty-six sites across 20 countries.
Patients: 200 treatment-naïve patients were randomized and exposed.
Interventions: Patients were randomized 2:1 to somapacitan (0.16 mg/kg/wk) or daily GH (Norditropin; 0.034 mg/kg/d), administered subcutaneously.
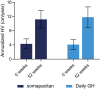
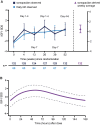
Main outcome measures: The primary endpoint was annualized height velocity (HV; cm/y) at week 52. Additional assessments included HV SD score (SDS), height SDS, bone age, IGF-I SDS, patient-reported outcomes, and safety measures.
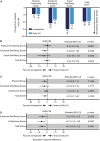
Results: Estimated mean HV at week 52 was 11.2 and 11.7 cm/y for somapacitan and daily GH, respectively. Noninferiority was confirmed. Changes in HV SDS, height SDS, bone age, and IGF-I SDS from baseline to week 52 were similar between treatment groups. At week 52, mean IGF-I SDS values were similar between treatment groups and within normal range (-2 to +2). Safety of somapacitan was consistent with the well-known daily GH profile. Low proportions of injection-site reactions were reported for somapacitan (5.3%) and daily GH (5.9%). Both treatments similarly reduced disease burden from baseline to week 52, whereas a greater treatment burden reduction was observed for somapacitan.
Conclusions: Similar efficacy for somapacitan compared to daily GH was demonstrated over 52 weeks of treatment with comparable safety and mean IGF-I SDS levels in treatment-naïve children with GHD.
Keywords: growth hormone; growth hormone deficiency; growth hormone replacement therapy; long-acting growth hormone; somapacitan; treatment burden.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

Comment in
-
Response to Letter to the Editor From Chatelain et al: "Weekly Somapacitan Is Effective and Well Tolerated in Children With GH Deficiency: The Randomized Phase 3 REAL4 Trial".J Clin Endocrinol Metab. 2023 Jul 14;108(8):e647-e648. doi: 10.1210/clinem/dgad095. J Clin Endocrinol Metab. 2023. PMID: 36869702 Free PMC article. Clinical Trial. No abstract available.
-
Letter to the Editor From Chatelain et al: "Weekly Somapacitan Is Effective and Well Tolerated in Children With GH Deficiency: The Randomized Phase 3 REAL4 Trial".J Clin Endocrinol Metab. 2023 Jul 14;108(8):e644-e645. doi: 10.1210/clinem/dgad096. J Clin Endocrinol Metab. 2023. PMID: 36869710 Free PMC article. Clinical Trial. No abstract available.
Similar articles
-
Effective GH Replacement With Somapacitan in Children With GHD: REAL4 2-year Results and After Switch From Daily GH.J Clin Endocrinol Metab. 2023 Nov 17;108(12):3090-3099. doi: 10.1210/clinem/dgad394. J Clin Endocrinol Metab. 2023. PMID: 37406251 Free PMC article. Clinical Trial.
-
Effective growth hormone replacement with once-weekly somapacitan in Japanese children with growth hormone deficiency: Results from REAL4, a phase 3 clinical trial.Clin Endocrinol (Oxf). 2024 Apr;100(4):389-398. doi: 10.1111/cen.15025. Epub 2024 Feb 18. Clin Endocrinol (Oxf). 2024. PMID: 38368603 Clinical Trial.
-
Once-Weekly Somapacitan vs Daily GH in Children With GH Deficiency: Results From a Randomized Phase 2 Trial.J Clin Endocrinol Metab. 2020 Apr 1;105(4):e1847-61. doi: 10.1210/clinem/dgz310. J Clin Endocrinol Metab. 2020. PMID: 31917835 Free PMC article. Clinical Trial.
-
Once-Weekly Somapacitan as an Alternative Management of Growth Hormone Deficiency in Prepubertal Children: A Systematic Review and Meta-Analysis of Randomized Controlled Trial.Children (Basel). 2024 Feb 9;11(2):227. doi: 10.3390/children11020227. Children (Basel). 2024. PMID: 38397339 Free PMC article. Review.
-
Utilizing Somapacitan, a Long-acting Growth Hormone Formulation, for the Treatment of Adult Growth Hormone Deficiency: A Guide for Clinicians.Endocr Pract. 2024 Oct;30(10):1003-1010. doi: 10.1016/j.eprac.2024.07.003. Epub 2024 Jul 9. Endocr Pract. 2024. PMID: 38992799 Review.
Cited by
-
What do we do now that the long-acting growth hormone is here?Front Endocrinol (Lausanne). 2022 Aug 22;13:980979. doi: 10.3389/fendo.2022.980979. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36072938 Free PMC article. Review.
-
Long-acting growth hormone in the treatment of growth hormone deficiency in children: a systematic literature review and network meta-analysis.Sci Rep. 2024 Apr 5;14(1):8061. doi: 10.1038/s41598-024-58616-4. Sci Rep. 2024. PMID: 38580693 Free PMC article.
-
Response to Letter to the Editor From Chatelain et al: "Weekly Somapacitan Is Effective and Well Tolerated in Children With GH Deficiency: The Randomized Phase 3 REAL4 Trial".J Clin Endocrinol Metab. 2023 Jul 14;108(8):e647-e648. doi: 10.1210/clinem/dgad095. J Clin Endocrinol Metab. 2023. PMID: 36869702 Free PMC article. Clinical Trial. No abstract available.
-
Model-Based Analysis of IGF-I Response, Dosing, and Monitoring for Once-Weekly Somapacitan in Children With GH Deficiency.J Endocr Soc. 2023 Sep 11;7(11):bvad115. doi: 10.1210/jendso/bvad115. eCollection 2023 Oct 9. J Endocr Soc. 2023. PMID: 37818403 Free PMC article.
-
Patients with Growth-Related Disorders and Caregivers Prefer the Somapacitan Device to the Somatrogon Device: Results from a Randomized Crossover Study Assessing Device Preference and Ease of Use Following Simulated Injections.Med Devices (Auckl). 2024 Oct 30;17:427-439. doi: 10.2147/MDER.S484354. eCollection 2024. Med Devices (Auckl). 2024. PMID: 39493440 Free PMC article.
References
-
- Grimberg A, DiVall SA, Polychronakos C, et al. . Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361–397. - PubMed
-
- Yuen KCJ, Miller BS, Biller BMK. The current state of long-acting growth hormone preparations for growth hormone therapy. Curr Opin Endocrinol Diabetes Obes. 2018;25(4):267–273. - PubMed
-
- Kapoor RR, Burke SA, Sparrow SE, et al. . Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93(2):147–148. - PubMed

