Effect of Vibrio-Derived Extracellular Protease vEP-45 on the Blood Complement System
- PMID: 34440030
- PMCID: PMC8389632
- DOI: 10.3390/biology10080798
Effect of Vibrio-Derived Extracellular Protease vEP-45 on the Blood Complement System
Abstract
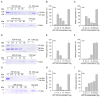
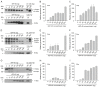
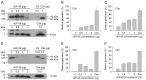
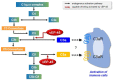
Vibrio vulnificus is a pathogenic bacterium that can causes wound infections and fetal septicemia. We have reported that V. vulnificus ATCC29307 produces an extracellular zinc-metalloprotease (named vEP-45). Our previous results showed that vEP-45 can convert prothrombin to active thrombin and also activate the plasma kallikrein/kinin system. In this study, the effect of vEP-45 on the activation of the complement system was examined. We found that vEP-45 could proteolytically convert the key complement precursor molecules, including C3, C4, and C5, to their corresponding active forms (e.g., C3a, C3b, C4a, C4b, and C5a) in vitro cleavage assays. C5b production from C5 cleavage mediated by vEP-45 was not observed, whereas the level of C5a was increased in a dose-dependent manner compared to that of the non-treated control. The cleavage of the complement proteins in human plasma by vEP-45 was also confirmed via Western blotting. Furthermore, vEP-45 could convert C3 and C5 to active C3a and C5a as a proinflammatory mediator, while no cleavage of C4 was observed. These results suggest that vEP-45 can activate the complement system involved in innate immunity through an alternative pathway.
Keywords: C3a; C5a; Vibrio vulnificus; complement system; innate immunity; vEP-45 protease.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures
Similar articles
-
Pleiotropic effects of a vibrio extracellular protease on the activation of contact system.Biochem Biophys Res Commun. 2014 Jul 25;450(2):1099-103. doi: 10.1016/j.bbrc.2014.06.121. Epub 2014 Jul 1. Biochem Biophys Res Commun. 2014. PMID: 24996182
-
C-ter100 peptide derived from Vibrio vEP-45 protease acts as a pathogen-associated molecular pattern to induce inflammation and innate immunity.PLoS Pathog. 2024 Aug 26;20(8):e1012474. doi: 10.1371/journal.ppat.1012474. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39186780 Free PMC article.
-
The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins C3a and C5a.J Immunol. 1995 Jul 1;155(1):266-74. J Immunol. 1995. PMID: 7602103
-
Pathways of complement activation in membranoproliferative glomerulonephritis and allograft rejection.Transplant Proc. 1977 Mar;9(1):729-39. Transplant Proc. 1977. PMID: 325806 Review.
-
Inhibiting the C5-C5a receptor axis.Mol Immunol. 2011 Aug;48(14):1631-42. doi: 10.1016/j.molimm.2011.04.014. Epub 2011 May 6. Mol Immunol. 2011. PMID: 21549429 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

