Fengycin A Analogues with Enhanced Chemical Stability and Antifungal Properties
- PMID: 34077216
- PMCID: PMC8289291
- DOI: 10.1021/acs.orglett.1c01387
Fengycin A Analogues with Enhanced Chemical Stability and Antifungal Properties
Abstract
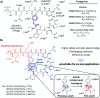
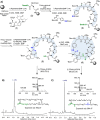
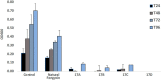
Fengycins are cyclic lipo-depsipeptides produced by Bacillus spp. that display potent antifungal properties but are chemically unstable. This instability has meant that no total synthesis of any fengycin has been published. Here we report the synthesis of fengycin A analogues that display enhanced antifungal properties and chemical stability under both basic and acidic conditions. The analogues prepared also demonstrate that the fengycin core structure can be modified and simplified without the loss of antifungal activity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Identification of cyclic lipopeptides produced by Bacillus vallismortis R2 and their antifungal activity against Alternaria alternata.J Appl Microbiol. 2017 Jan;122(1):139-152. doi: 10.1111/jam.13303. Epub 2016 Nov 23. J Appl Microbiol. 2017. PMID: 27665751
-
A Novel Variant of Narrow-Spectrum Antifungal Bacterial Lipopeptides That Strongly Inhibit Ganoderma boninense.Probiotics Antimicrob Proteins. 2018 Mar;10(1):110-117. doi: 10.1007/s12602-017-9334-2. Probiotics Antimicrob Proteins. 2018. PMID: 29101528
-
Synthesis and antifungal activities of cyclic octa-lipopeptide burkholdine analogues.Bioorg Med Chem Lett. 2013 Jul 15;23(14):4244-7. doi: 10.1016/j.bmcl.2013.04.091. Epub 2013 May 14. Bioorg Med Chem Lett. 2013. PMID: 23769641
-
Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics.Biomed Res Int. 2015;2015:473050. doi: 10.1155/2015/473050. Epub 2015 Jan 6. Biomed Res Int. 2015. PMID: 25632392 Free PMC article. Review.
-
Biological activity of lipopeptides from Bacillus.Appl Microbiol Biotechnol. 2017 Aug;101(15):5951-5960. doi: 10.1007/s00253-017-8396-0. Epub 2017 Jul 6. Appl Microbiol Biotechnol. 2017. PMID: 28685194 Review.
Cited by
-
Pseudomonas beijingensis sp. nov., a novel species widely colonizing plant rhizosphere.Int J Syst Evol Microbiol. 2024 Jul;74(7):006473. doi: 10.1099/ijsem.0.006473. Int J Syst Evol Microbiol. 2024. PMID: 39058535
-
Genetic Evidences of Biosurfactant Production in Two Bacillus subtilis Strains MB415 and MB418 Isolated From Oil Contaminated Soil.Front Bioeng Biotechnol. 2022 Apr 26;10:855762. doi: 10.3389/fbioe.2022.855762. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35557861 Free PMC article.
-
Synthetic studies with the brevicidine and laterocidine lipopeptide antibiotics including analogues with enhanced properties and in vivo efficacy.Chem Sci. 2022 Feb 23;13(12):3563-3570. doi: 10.1039/d2sc00143h. eCollection 2022 Mar 24. Chem Sci. 2022. PMID: 35432860 Free PMC article.
-
Design of Synthetic Surrogates for the Macrolactone Linker Motif in Coibamide A.ACS Med Chem Lett. 2023 Sep 19;14(10):1344-1350. doi: 10.1021/acsmedchemlett.3c00232. eCollection 2023 Oct 12. ACS Med Chem Lett. 2023. PMID: 37849553 Free PMC article.
-
Efficacy of cyclic lipopeptides obtained from Bacillus subtilis to inhibit the growth of Microsporum canis isolated from cats.Heliyon. 2021 Sep 11;7(9):e07980. doi: 10.1016/j.heliyon.2021.e07980. eCollection 2021 Sep. Heliyon. 2021. PMID: 34585007 Free PMC article.
References
-
- Cawoy H.; Bettiol W.; Fickers P.; Ongena M.. Bacillus-Based Biological Control of Plant Diseases. In Pesticides in the Modern World—Pesticides Use and Management; IntechOpen, 2011.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

