Comprehensive integrative analyses identify GLT8D1 and CSNK2B as schizophrenia risk genes
- PMID: 29483533
- PMCID: PMC5826945
- DOI: 10.1038/s41467-018-03247-3
Comprehensive integrative analyses identify GLT8D1 and CSNK2B as schizophrenia risk genes
Erratum in
-
Author Correction: Comprehensive integrative analyses identify GLT8D1 and CSNK2B as schizophrenia risk genes.Nat Commun. 2018 Nov 16;9(1):4905. doi: 10.1038/s41467-018-07401-9. Nat Commun. 2018. PMID: 30446647 Free PMC article.
Abstract
Recent genome-wide association studies (GWAS) have identified multiple risk loci that show strong associations with schizophrenia. However, pinpointing the potential causal genes at the reported loci remains a major challenge. Here we identify candidate causal genes for schizophrenia using an integrative genomic approach. Sherlock integrative analysis shows that ALMS1, GLT8D1, and CSNK2B are schizophrenia risk genes, which are validated using independent brain expression quantitative trait loci (eQTL) data and integrative analysis method (SMR). Consistently, gene expression analysis in schizophrenia cases and controls further supports the potential role of these three genes in the pathogenesis of schizophrenia. Finally, we show that GLT8D1 and CSNK2B knockdown promote the proliferation and inhibit the differentiation abilities of neural stem cells, and alter morphology and synaptic transmission of neurons. These convergent lines of evidence suggest that the ALMS1, CSNK2B, and GLT8D1 genes may be involved in pathophysiology of schizophrenia.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
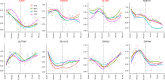

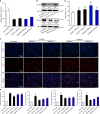
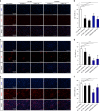
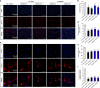
Similar articles
-
Convergent lines of evidence support NOTCH4 as a schizophrenia risk gene.J Med Genet. 2021 Oct;58(10):666-678. doi: 10.1136/jmedgenet-2020-106830. Epub 2020 Sep 8. J Med Genet. 2021. PMID: 32900838
-
Integrative Analyses Followed by Functional Characterization Reveal TMEM180 as a Schizophrenia Risk Gene.Schizophr Bull. 2021 Aug 21;47(5):1364-1374. doi: 10.1093/schbul/sbab032. Schizophr Bull. 2021. PMID: 33768244 Free PMC article.
-
Identification of the primate-specific gene BTN3A2 as an additional schizophrenia risk gene in the MHC loci.EBioMedicine. 2019 Jun;44:530-541. doi: 10.1016/j.ebiom.2019.05.006. Epub 2019 May 24. EBioMedicine. 2019. PMID: 31133542 Free PMC article.
-
Genome-wide expression quantitative trait loci analysis in asthma.Curr Opin Allergy Clin Immunol. 2013 Oct;13(5):487-94. doi: 10.1097/ACI.0b013e328364e951. Curr Opin Allergy Clin Immunol. 2013. PMID: 23945176 Review.
-
Path from schizophrenia genomics to biology: gene regulation and perturbation in neurons derived from induced pluripotent stem cells and genome editing.Neurosci Bull. 2015 Feb;31(1):113-27. doi: 10.1007/s12264-014-1488-2. Epub 2015 Jan 9. Neurosci Bull. 2015. PMID: 25575480 Free PMC article. Review.
Cited by
-
TWAS Atlas: a curated knowledgebase of transcriptome-wide association studies.Nucleic Acids Res. 2023 Jan 6;51(D1):D1179-D1187. doi: 10.1093/nar/gkac821. Nucleic Acids Res. 2023. PMID: 36243959 Free PMC article.
-
Recent advances in the diagnosis and prognosis of amyotrophic lateral sclerosis.Lancet Neurol. 2022 May;21(5):480-493. doi: 10.1016/S1474-4422(21)00465-8. Epub 2022 Mar 22. Lancet Neurol. 2022. PMID: 35334233 Free PMC article. Review.
-
Genome-wide meta-analyses reveal novel loci for verbal short-term memory and learning.Mol Psychiatry. 2022 Nov;27(11):4419-4431. doi: 10.1038/s41380-022-01710-8. Epub 2022 Aug 16. Mol Psychiatry. 2022. PMID: 35974141 Free PMC article.
-
Transcriptome-wide association study identifies new susceptibility genes and pathways for depression.Transl Psychiatry. 2021 May 21;11(1):306. doi: 10.1038/s41398-021-01411-w. Transl Psychiatry. 2021. PMID: 34021117 Free PMC article.
-
Mutations in the Glycosyltransferase Domain of GLT8D1 Are Associated with Familial Amyotrophic Lateral Sclerosis.Cell Rep. 2019 Feb 26;26(9):2298-2306.e5. doi: 10.1016/j.celrep.2019.02.006. Cell Rep. 2019. PMID: 30811981 Free PMC article.
References
-
- Andreasen NC, Black DW. Introductory Textbook of Psychiatry. 4th edn. Washington, DC: American Psychiatric Publishing, Inc.; 2006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

