Metatranscriptome analysis reveals host-microbiome interactions in traps of carnivorous Genlisea species
- PMID: 26236284
- PMCID: PMC4500957
- DOI: 10.3389/fmicb.2015.00526
Metatranscriptome analysis reveals host-microbiome interactions in traps of carnivorous Genlisea species
Abstract
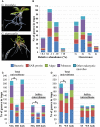
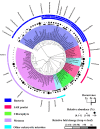
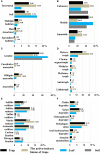
In the carnivorous plant genus Genlisea a unique lobster pot trapping mechanism supplements nutrition in nutrient-poor habitats. A wide spectrum of microbes frequently occurs in Genlisea's leaf-derived traps without clear relevance for Genlisea carnivory. We sequenced the metatranscriptomes of subterrestrial traps vs. the aerial chlorophyll-containing leaves of G. nigrocaulis and of G. hispidula. Ribosomal RNA assignment revealed soil-borne microbial diversity in Genlisea traps, with 92 genera of 19 phyla present in more than one sample. Microbes from 16 of these phyla including proteobacteria, green algae, amoebozoa, fungi, ciliates and metazoans, contributed additionally short-lived mRNA to the metatranscriptome. Furthermore, transcripts of 438 members of hydrolases (e.g., proteases, phosphatases, lipases), mainly resembling those of metazoans, ciliates and green algae, were found. Compared to aerial leaves, Genlisea traps displayed a transcriptional up-regulation of endogenous NADH oxidases generating reactive oxygen species as well as of acid phosphatases for prey digestion. A leaf-vs.-trap transcriptome comparison reflects that carnivory provides inorganic P- and different forms of N-compounds (ammonium, nitrate, amino acid, oligopeptides) and implies the need to protect trap cells against oxidative stress. The analysis elucidates a complex food web inside the Genlisea traps, and suggests ecological relationships between this plant genus and its entrapped microbiome.
Keywords: Genlisea; RNA-sequencing; algae commensalism; lobster pot trapping; metatranscriptomics; plant carnivory; plant-microbe interaction; whole-genome gene transcription analysis.
Figures
Similar articles
-
Chromatin organization and cytological features of carnivorous Genlisea species with large genome size differences.Front Plant Sci. 2015 Aug 20;6:613. doi: 10.3389/fpls.2015.00613. eCollection 2015. Front Plant Sci. 2015. PMID: 26347752 Free PMC article.
-
Oxygen concentrations inside the traps of the carnivorous plants Utricularia and Genlisea (Lentibulariaceae).Ann Bot. 2007 Oct;100(4):849-56. doi: 10.1093/aob/mcm182. Epub 2007 Aug 23. Ann Bot. 2007. PMID: 17720681 Free PMC article.
-
Structural gradients and anisotropic hydraulic conductivity in the enigmatic eel traps of carnivorous corkscrew plants (Genlisea spp.).Am J Bot. 2021 Dec;108(12):2356-2370. doi: 10.1002/ajb2.1779. Epub 2021 Dec 24. Am J Bot. 2021. PMID: 34648183
-
Energetics and the evolution of carnivorous plants--Darwin's 'most wonderful plants in the world'.J Exp Bot. 2009;60(1):19-42. doi: 10.1093/jxb/ern179. J Exp Bot. 2009. PMID: 19213724 Review.
-
On the Origin of Carnivory: Molecular Physiology and Evolution of Plants on an Animal Diet.Annu Rev Plant Biol. 2021 Jun 17;72:133-153. doi: 10.1146/annurev-arplant-080620-010429. Epub 2021 Jan 12. Annu Rev Plant Biol. 2021. PMID: 33434053 Review.
Cited by
-
Chromatin organization and cytological features of carnivorous Genlisea species with large genome size differences.Front Plant Sci. 2015 Aug 20;6:613. doi: 10.3389/fpls.2015.00613. eCollection 2015. Front Plant Sci. 2015. PMID: 26347752 Free PMC article.
-
An acidophilic fungus promotes prey digestion in a carnivorous plant.Nat Microbiol. 2024 Oct;9(10):2522-2537. doi: 10.1038/s41564-024-01766-y. Epub 2024 Aug 1. Nat Microbiol. 2024. PMID: 39090391 Free PMC article.
-
Analysis of the mouse gut microbiome using full-length 16S rRNA amplicon sequencing.Sci Rep. 2016 Jul 14;6:29681. doi: 10.1038/srep29681. Sci Rep. 2016. PMID: 27411898 Free PMC article.
-
Venus flytrap carnivorous lifestyle builds on herbivore defense strategies.Genome Res. 2016 Jun;26(6):812-25. doi: 10.1101/gr.202200.115. Epub 2016 May 4. Genome Res. 2016. PMID: 27197216 Free PMC article.
-
The Metagenome of Utricularia gibba's Traps: Into the Microbial Input to a Carnivorous Plant.PLoS One. 2016 Feb 9;11(2):e0148979. doi: 10.1371/journal.pone.0148979. eCollection 2016. PLoS One. 2016. PMID: 26859489 Free PMC article.
References
-
- Adamec L. (1997). Mineral nutrition of carnivorous plants: a review. Bot. Rev. 63, 273–299. 10.1007/BF02857953 - DOI
-
- Adamec L. (2003). Zero water flows in Genlisea traps. Carniv. Pl. Newslett. 32, 46–48.
LinkOut - more resources
Full Text Sources
Other Literature Sources

