Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: a comparison of docetaxel-loaded block copolymer micelles and Taxotere®
- PMID: 23626842
- PMCID: PMC3633836
- DOI: 10.1371/journal.pone.0062630
Multicellular tumor spheroids for evaluation of cytotoxicity and tumor growth inhibitory effects of nanomedicines in vitro: a comparison of docetaxel-loaded block copolymer micelles and Taxotere®
Abstract
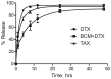
While 3-D tissue models have received increasing attention over the past several decades in the development of traditional anti-cancer therapies, their potential application for the evaluation of advanced drug delivery systems such as nanomedicines has been largely overlooked. In particular, new insight into drug resistance associated with the 3-D tumor microenvironment has called into question the validity of 2-D models for prediction of in vivo anti-tumor activity. In this work, a series of complementary assays was established for evaluating the in vitro efficacy of docetaxel (DTX) -loaded block copolymer micelles (BCM+DTX) and Taxotere® in 3-D multicellular tumor spheroid (MCTS) cultures. Spheroids were found to be significantly more resistant to treatment than monolayer cultures in a cell line dependent manner. Limitations in treatment efficacy were attributed to mechanisms of resistance associated with properties of the spheroid microenvironment. DTX-loaded micelles demonstrated greater therapeutic effect in both monolayer and spheroid cultures in comparison to Taxotere®. Overall, this work demonstrates the use of spheroids as a viable platform for the evaluation of nanomedicines in conditions which more closely reflect the in vivo tumor microenvironment relative to traditional monolayer cultures. By adaptation of traditional cell-based assays, spheroids have the potential to serve as intermediaries between traditional in vitro and in vivo models for high-throughput assessment of therapeutic candidates.
Conflict of interest statement
Figures

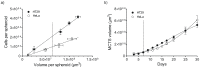

Similar articles
-
Redox-responsive polymeric micelles formed by conjugating gambogic acid with bioreducible poly(amido amine)s for the co-delivery of docetaxel and MMP-9 shRNA.Acta Biomater. 2018 Mar 1;68:137-153. doi: 10.1016/j.actbio.2017.12.028. Epub 2017 Dec 26. Acta Biomater. 2018. PMID: 29288085
-
Enhanced antitumor efficacy and decreased toxicity by self-associated docetaxel in phospholipid-based micelles.Int J Pharm. 2012 Sep 15;434(1-2):413-9. doi: 10.1016/j.ijpharm.2012.06.014. Epub 2012 Jun 12. Int J Pharm. 2012. PMID: 22698861
-
In vitro and in vivo evaluation of docetaxel-loaded stearic acid-modified Bletilla striata polysaccharide copolymer micelles.PLoS One. 2017 Mar 23;12(3):e0173172. doi: 10.1371/journal.pone.0173172. eCollection 2017. PLoS One. 2017. PMID: 28334044 Free PMC article.
-
Intelligent polymeric micelles: development and application as drug delivery for docetaxel.J Drug Target. 2017 Apr;25(4):285-295. doi: 10.1080/1061186X.2016.1245309. Epub 2016 Oct 28. J Drug Target. 2017. PMID: 27701892 Review.
-
Kinetics of Nanomedicine in Tumor Spheroid as an In Vitro Model System for Efficient Tumor-Targeted Drug Delivery With Insights From Mathematical Models.Front Bioeng Biotechnol. 2021 Dec 1;9:785937. doi: 10.3389/fbioe.2021.785937. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34926430 Free PMC article. Review.
Cited by
-
Caffeic Acid, Quercetin and 5-Fluorocytidine-Functionalized Au-Fe3O4 Nanoheterodimers for X-ray-Triggered Drug Delivery in Breast Tumor Spheroids.Nanomaterials (Basel). 2021 Apr 29;11(5):1167. doi: 10.3390/nano11051167. Nanomaterials (Basel). 2021. PMID: 33947086 Free PMC article.
-
Development of constitutively synergistic nanoformulations to enhance chemosensitivity in T-cell leukemia.J Control Release. 2023 Sep;361:470-482. doi: 10.1016/j.jconrel.2023.07.045. Epub 2023 Aug 14. J Control Release. 2023. PMID: 37543290 Free PMC article.
-
Three-Dimensional Spheroids for Cancer Research.Methods Mol Biol. 2023;2645:65-103. doi: 10.1007/978-1-0716-3056-3_3. Methods Mol Biol. 2023. PMID: 37202612
-
Three-Dimensional Tumor Spheroids as a Tool for Reliable Investigation of Combined Gold Nanoparticle and Docetaxel Treatment.Cancers (Basel). 2021 Mar 23;13(6):1465. doi: 10.3390/cancers13061465. Cancers (Basel). 2021. PMID: 33806801 Free PMC article.
-
Moving beyond traditional therapies: the role of nanomedicines in lung cancer.Front Pharmacol. 2024 Feb 8;15:1363346. doi: 10.3389/fphar.2024.1363346. eCollection 2024. Front Pharmacol. 2024. PMID: 38389925 Free PMC article. Review.
References
-
- Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99: 1441–1454. - PubMed
-
- Minchinton AI, Tannock IF (2006) Drug penetration in solid tumours. Nat Rev Cancer 6: 583–592. - PubMed
-
- Vaupel P, Mayer A (2007) Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev 26: 225–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

