The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection
- PMID: 22792067
- PMCID: PMC3390405
- DOI: 10.1371/journal.ppat.1002793
The lectin pathway of complement activation is a critical component of the innate immune response to pneumococcal infection
Abstract
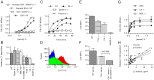
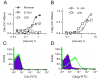
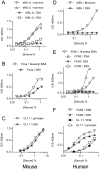
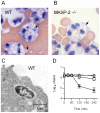
The complement system plays a key role in host defense against pneumococcal infection. Three different pathways, the classical, alternative and lectin pathways, mediate complement activation. While there is limited information available on the roles of the classical and the alternative activation pathways of complement in fighting streptococcal infection, little is known about the role of the lectin pathway, mainly due to the lack of appropriate experimental models of lectin pathway deficiency. We have recently established a mouse strain deficient of the lectin pathway effector enzyme mannan-binding lectin associated serine protease-2 (MASP-2) and shown that this mouse strain is unable to form the lectin pathway specific C3 and C5 convertases. Here we report that MASP-2 deficient mice (which can still activate complement via the classical pathway and the alternative pathway) are highly susceptible to pneumococcal infection and fail to opsonize Streptococcus pneumoniae in the none-immune host. This defect in complement opsonisation severely compromises pathogen clearance in the lectin pathway deficient host. Using sera from mice and humans with defined complement deficiencies, we demonstrate that mouse ficolin A, human L-ficolin, and collectin 11 in both species, but not mannan-binding lectin (MBL), are the pattern recognition molecules that drive lectin pathway activation on the surface of S. pneumoniae. We further show that pneumococcal opsonisation via the lectin pathway can proceed in the absence of C4. This study corroborates the essential function of MASP-2 in the lectin pathway and highlights the importance of MBL-independent lectin pathway activation in the host defense against pneumococci.
Conflict of interest statement
I have read the journal's policy and have the following conflicts: Thomas Dudler is an employee of Omeros Inc., which aims to market anti-MASP-2 monoclonal antibodies for therapeutic use. Thomas Dudler, Wilhelm J. Schwaeble, Cordula M. Stover and Teizo Fujita are named inventors on patents held by the University of Leicester, protecting the I.P. behind the therapeutic application of MASP-2 specific inhibitors for treating lectin pathway mediated inflammatory pathologies. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures
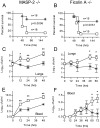
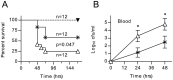
Similar articles
-
Mice deficient in ficolin, a lectin complement pathway recognition molecule, are susceptible to Streptococcus pneumoniae infection.J Immunol. 2012 Dec 15;189(12):5860-6. doi: 10.4049/jimmunol.1200836. Epub 2012 Nov 12. J Immunol. 2012. PMID: 23150716
-
Mannose-binding lectin (MBL)-associated serine protease (MASP)-1 contributes to activation of the lectin complement pathway.J Immunol. 2008 May 1;180(9):6132-8. doi: 10.4049/jimmunol.180.9.6132. J Immunol. 2008. PMID: 18424734
-
A journey through the lectin pathway of complement-MBL and beyond.Immunol Rev. 2016 Nov;274(1):74-97. doi: 10.1111/imr.12468. Immunol Rev. 2016. PMID: 27782323 Review.
-
Essential role of mannose-binding lectin-associated serine protease-1 in activation of the complement factor D.J Exp Med. 2010 Jan 18;207(1):29-37. doi: 10.1084/jem.20090633. Epub 2009 Dec 28. J Exp Med. 2010. PMID: 20038603 Free PMC article.
-
Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway.Adv Exp Med Biol. 2007;598:93-104. doi: 10.1007/978-0-387-71767-8_8. Adv Exp Med Biol. 2007. PMID: 17892207 Review.
Cited by
-
The relative merits of therapies being developed to tackle inappropriate ('self'-directed) complement activation.Auto Immun Highlights. 2016 Dec;7(1):6. doi: 10.1007/s13317-016-0078-x. Epub 2016 Mar 3. Auto Immun Highlights. 2016. PMID: 26935316 Free PMC article.
-
Complement Recognition Pathways in Renal Transplantation.J Am Soc Nephrol. 2017 Sep;28(9):2571-2578. doi: 10.1681/ASN.2017010079. Epub 2017 Jun 29. J Am Soc Nephrol. 2017. PMID: 28663231 Free PMC article. Review.
-
Mannan binding lectin-associated serine protease-2 (MASP-2) critically contributes to post-ischemic brain injury independent of MASP-1.J Neuroinflammation. 2016 Aug 30;13(1):213. doi: 10.1186/s12974-016-0684-6. J Neuroinflammation. 2016. PMID: 27577570 Free PMC article.
-
Complement-Coagulation Cross-Talk: A Potential Mediator of the Physiological Activation of Complement by Low pH.Front Immunol. 2015 May 6;6:215. doi: 10.3389/fimmu.2015.00215. eCollection 2015. Front Immunol. 2015. PMID: 25999953 Free PMC article. Review.
-
Ficolin-2 Lectin Complement Pathway Mediates Capsule-Specific Innate Immunity Against Invasive Pneumococcal Disease.Front Immunol. 2022 Mar 28;13:841062. doi: 10.3389/fimmu.2022.841062. eCollection 2022. Front Immunol. 2022. PMID: 35418983 Free PMC article.
References
-
- Miller E, Waight P, Efstratiou A, Brisson M, Johnson A, et al. Epidemiology of invasive and other pneumococcal disease in children in england and wales 1996–1998. Acta Paediatr. 2000;Suppl 89:11–16. - PubMed
-
- Kyaw MH, Christie P, Clarke SC, Mooney JD, Ahmed S, et al. Invasive pneumococcal disease in scotland, 1999–2001: Use of record linkage to explore associations between patients and disease in relation to future vaccination policy. Clin Infect Dis. 2003;37:1283–1291. - PubMed
-
- Bruyn GA, Zegers BJ, van Furth R. Mechanisms of host defense against infection with streptococcus pneumoniae. Clin Infect Dis. 1992;14:251–262. - PubMed
-
- Schwaeble W, Dahl MR, Thiel S, Stover C, Jensenius JC. The mannan-binding lectin-associated serine proteases (MASPs) and MAp19: Four components of the lectin pathway activation complex encoded by two genes. Immunobiology. 2002;205:455–466. - PubMed
-
- Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

