Molecular mechanisms involved in farnesol-induced apoptosis
- PMID: 19520495
- PMCID: PMC2815016
- DOI: 10.1016/j.canlet.2009.05.015
Molecular mechanisms involved in farnesol-induced apoptosis
Abstract
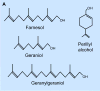
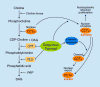
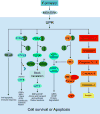
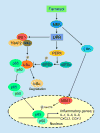
The isoprenoid alcohol farnesol is an effective inducer of cell cycle arrest and apoptosis in a variety of carcinoma cell types. In addition, farnesol has been reported to inhibit tumorigenesis in several animal models suggesting that it functions as a chemopreventative and anti-tumor agent in vivo. A number of different biochemical and cellular processes have been implicated in the growth-inhibitory and apoptosis-inducing effects of farnesol. These include regulation of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and CTP:phosphocholine cytidylyltransferase alpha (CCTalpha), rate-limiting enzymes in the mevalonate pathway and phosphatidylcholine biosynthesis, respectively, and the generation of reactive oxygen species. In some cell types the action of farnesol is mediated through nuclear receptors, including activation of farnesoid X receptor (FXR) and peroxisome proliferator-activated receptors (PPARs). Recent studies have revealed that induction of endoplasmic reticulum (ER) stress and the subsequent activation of the unfolded protein response (UPR) play a critical role in the induction of apoptosis by farnesol in lung carcinoma cells. This induction was found to be dependent on the activation of the MEK1/2-ERK1/2 pathway. In addition, farnesol induces activation of the NF-kappaB signaling pathway and a number of NF-kappaB target genes. Optimal activation of NF-kappaB was reported to depend on the phosphorylation of p65/RelA by the MEK1/2-MSK1 signaling pathway. In a number of cells farnesol-induced apoptosis was found to be linked to activation of the apoptosome. This review provides an overview of the biochemical and cellular processes regulated by farnesol in relationship to its growth-inhibitory, apoptosis-promoting, and anti-tumor effects.
Published by Elsevier Ireland Ltd.
Conflict of interest statement
None declared.
Figures

Similar articles
-
NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway.J Biol Chem. 2008 Jun 13;283(24):16391-9. doi: 10.1074/jbc.M800945200. Epub 2008 Apr 18. J Biol Chem. 2008. PMID: 18424438 Free PMC article.
-
Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response.Cancer Res. 2007 Aug 15;67(16):7929-36. doi: 10.1158/0008-5472.CAN-07-0931. Cancer Res. 2007. PMID: 17699800
-
Competitive inhibition of choline phosphotransferase by geranylgeraniol and farnesol inhibits phosphatidylcholine synthesis and induces apoptosis in human lung adenocarcinoma A549 cells.J Biol Chem. 1998 Oct 2;273(40):26179-86. doi: 10.1074/jbc.273.40.26179. J Biol Chem. 1998. PMID: 9748300
-
Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention.Exp Biol Med (Maywood). 2004 Jul;229(7):567-85. doi: 10.1177/153537020422900701. Exp Biol Med (Maywood). 2004. PMID: 15229351 Review.
-
Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response.Free Radic Biol Med. 2013 Dec;65:162-174. doi: 10.1016/j.freeradbiomed.2013.06.020. Epub 2013 Jun 19. Free Radic Biol Med. 2013. PMID: 23792277 Review.
Cited by
-
Juvenile Hormone Biosynthesis in Insects: What Is New, What Do We Know, and What Questions Remain?Int Sch Res Notices. 2014 Oct 19;2014:967361. doi: 10.1155/2014/967361. eCollection 2014. Int Sch Res Notices. 2014. PMID: 27382622 Free PMC article. Review.
-
Untargeted Metabolomic Characterization of Ovarian Tumors.Cancers (Basel). 2020 Dec 4;12(12):3642. doi: 10.3390/cancers12123642. Cancers (Basel). 2020. PMID: 33291756 Free PMC article.
-
Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T lymphoblastic leukemia Molt4 cells.Biochem Pharmacol. 2015 Oct 1;97(3):256-68. doi: 10.1016/j.bcp.2015.08.086. Epub 2015 Aug 11. Biochem Pharmacol. 2015. PMID: 26275811 Free PMC article.
-
The role of fatty aldehyde dehydrogenase in epidermal structure and function.Dermatoendocrinol. 2011 Apr;3(2):91-9. doi: 10.4161/derm.3.2.14619. Epub 2011 Apr 1. Dermatoendocrinol. 2011. PMID: 21695018 Free PMC article.
-
Synthesis of Zinc Oxide (ZnO)-Titanium Dioxide (TiO2)-Chitosan-Farnesol Nanocomposites and Assessment of Their Anticancer Potential in Human Leukemic MOLT-4 Cell Line.Bioinorg Chem Appl. 2022 Sep 28;2022:5949086. doi: 10.1155/2022/5949086. eCollection 2022. Bioinorg Chem Appl. 2022. Retraction in: Bioinorg Chem Appl. 2024 Jan 24;2024:9821307. doi: 10.1155/2024/9821307. PMID: 36212987 Free PMC article. Retracted.
References
-
- Hanley K, Komuves LG, Ng DC, Schoonjans K, He SS, Lau P, Bikle DD, Williams ML, Elias PM, Auwerx J, Feingold KR. Farnesol stimulates differentiation in epidermal keratinocytes via PPARalpha. J Biol Chem. 2000;275:11484–11491. - PubMed
-
- Rioja A, Pizzey AR, Marson CM, Thomas NS. Preferential induction of apoptosis of leukaemic cells by farnesol. FEBS Lett. 2000;467:291–295. - PubMed
-
- Bifulco M. Role of the isoprenoid pathway in ras transforming activity, cytoskeleton organization, cell proliferation and apoptosis. Life Sci. 2005;77:1740–1749. - PubMed
-
- Miquel K, Pradines A, Favre G. Farnesol and geranylgeraniol induce actin cytoskeleton disorganization and apoptosis in A549 lung adenocarcinoma cells. Biochem Biophys Res Commun. 1996;225:869–876. - PubMed
-
- Wright MM, Henneberry AL, Lagace TA, Ridgway ND, McMaster CR. Uncoupling farnesol-induced apoptosis from its inhibition of phosphatidylcholine synthesis. J Biol Chem. 2001;276:25254–25261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

