Polyphosphate-dependent synthesis of ATP and ADP by the family-2 polyphosphate kinases in bacteria
- PMID: 19001261
- PMCID: PMC2584756
- DOI: 10.1073/pnas.0807563105
Polyphosphate-dependent synthesis of ATP and ADP by the family-2 polyphosphate kinases in bacteria
Abstract
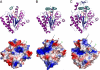
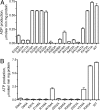
Inorganic polyphosphate (polyP) is a linear polymer of tens or hundreds of phosphate residues linked by high-energy bonds. It is found in all organisms and has been proposed to serve as an energy source in a pre-ATP world. This ubiquitous and abundant biopolymer plays numerous and vital roles in metabolism and regulation in prokaryotes and eukaryotes, but the underlying molecular mechanisms for most activities of polyP remain unknown. In prokaryotes, the synthesis and utilization of polyP are catalyzed by 2 families of polyP kinases, PPK1 and PPK2, and polyphosphatases. Here, we present structural and functional characterization of the PPK2 family. Proteins with a single PPK2 domain catalyze polyP-dependent phosphorylation of ADP to ATP, whereas proteins containing 2 fused PPK2 domains phosphorylate AMP to ADP. Crystal structures of 2 representative proteins, SMc02148 from Sinorhizobium meliloti and PA3455 from Pseudomonas aeruginosa, revealed a 3-layer alpha/beta/alpha sandwich fold with an alpha-helical lid similar to the structures of microbial thymidylate kinases, suggesting that these proteins share a common evolutionary origin and catalytic mechanism. Alanine replacement mutagenesis identified 9 conserved residues, which are required for activity and include the residues from both Walker A and B motifs and the lid. Thus, the PPK2s represent a molecular mechanism, which potentially allow bacteria to use polyP as an intracellular energy reserve for the generation of ATP and survival.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

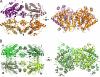

Similar articles
-
Rational design of substrate binding pockets in polyphosphate kinase for use in cost-effective ATP-dependent cascade reactions.Appl Microbiol Biotechnol. 2017 Jul;101(13):5325-5332. doi: 10.1007/s00253-017-8268-7. Epub 2017 Apr 18. Appl Microbiol Biotechnol. 2017. PMID: 28417169
-
Substrate recognition and mechanism revealed by ligand-bound polyphosphate kinase 2 structures.Proc Natl Acad Sci U S A. 2018 Mar 27;115(13):3350-3355. doi: 10.1073/pnas.1710741115. Epub 2018 Mar 12. Proc Natl Acad Sci U S A. 2018. PMID: 29531036 Free PMC article.
-
A new subfamily of polyphosphate kinase 2 (class III PPK2) catalyzes both nucleoside monophosphate phosphorylation and nucleoside diphosphate phosphorylation.Appl Environ Microbiol. 2014 Apr;80(8):2602-8. doi: 10.1128/AEM.03971-13. Epub 2014 Feb 14. Appl Environ Microbiol. 2014. PMID: 24532069 Free PMC article.
-
Polyphosphate Kinase 2 (PPK2) Enzymes: Structure, Function, and Roles in Bacterial Physiology and Virulence.Int J Mol Sci. 2022 Jan 8;23(2):670. doi: 10.3390/ijms23020670. Int J Mol Sci. 2022. PMID: 35054854 Free PMC article. Review.
-
Inorganic polyphosphate in the origin and survival of species.Proc Natl Acad Sci U S A. 2004 Nov 16;101(46):16085-7. doi: 10.1073/pnas.0406909101. Epub 2004 Nov 1. Proc Natl Acad Sci U S A. 2004. PMID: 15520374 Free PMC article. Review.
Cited by
-
Horizontal transfer of bacterial polyphosphate kinases to eukaryotes: implications for the ice age and land colonisation.BMC Res Notes. 2013 Jun 5;6:221. doi: 10.1186/1756-0500-6-221. BMC Res Notes. 2013. PMID: 23738841 Free PMC article.
-
MglA/SspA complex interactions are modulated by inorganic polyphosphate.PLoS One. 2013 Oct 8;8(10):e76428. doi: 10.1371/journal.pone.0076428. eCollection 2013. PLoS One. 2013. PMID: 24116108 Free PMC article.
-
Structural organization of biocatalytic systems: the next dimension of synthetic metabolism.Emerg Top Life Sci. 2019 Nov 11;3(5):579-586. doi: 10.1042/ETLS20190015. Emerg Top Life Sci. 2019. PMID: 33523157 Free PMC article.
-
Accumulation of inorganic polyphosphate enables stress endurance and catalytic vigour in Pseudomonas putida KT2440.Microb Cell Fact. 2013 May 20;12:50. doi: 10.1186/1475-2859-12-50. Microb Cell Fact. 2013. PMID: 23687963 Free PMC article.
-
A Modular In Vitro Platform for the Production of Terpenes and Polyketides from CO2.Angew Chem Int Ed Engl. 2021 Jul 19;60(30):16420-16425. doi: 10.1002/anie.202102333. Epub 2021 Jun 3. Angew Chem Int Ed Engl. 2021. PMID: 33938102 Free PMC article.
References
-
- Kornberg A, Rao NN, Ault-Riche D. Inorganic polyphosphate: A molecule of many functions. Annu Rev Biochem. 1999;68:89–125. - PubMed
-
- Kulaev IS, Vagabov VM. Polyphosphate metabolism in microorganisms. Adv Microb Physiol. 1983;24:83–171. - PubMed
-
- Brown MR, Kornberg A. The long and short of it: Polyphosphate, PPK, and bacterial survival. Trends Biochem Sci. 2008;33:284–290. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

