KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation
- PMID: 17245405
- PMCID: PMC2013306
- DOI: 10.1038/ncpcardio0792
KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation
Abstract
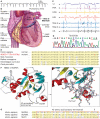
Background: A 53-year-old female presented with a 10-year history of paroxysmal atrial fibrillation (AF), precipitated by activity and refractory to medical therapy. In the absence of traditional risk factors for disease, a genetic defect in electrical homeostasis underlying stress-induced AF was explored.
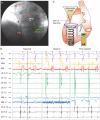
Investigations: Echocardiography, cardiac perfusion stress imaging, invasive electrophysiology with isoproterenol provocation, genomic DNA sequencing of K(ATP) channel genes, exclusion of mutation in 2,000 individuals free of AF, reconstitution of channel defect with molecular phenotyping, and verification of pathogenic link in targeted knockout.
Diagnosis: K(ATP) channelopathy caused by missense mutation (Thr1547Ile) of the ABCC9 gene conferring predisposition to adrenergic AF originating from the vein of Marshall.
Management: Disruption of arrhythmogenic gene-environment substrate at the vein of Marshall by radiofrequency ablation.
Figures

Similar articles
-
[Long term outcome of atrial fibrillation patients with KCNA5 and NPPA mutations post circumferential pulmonary vein ablation].Zhonghua Xin Xue Guan Bing Za Zhi. 2013 May;41(5):387-9. Zhonghua Xin Xue Guan Bing Za Zhi. 2013. PMID: 24021120 Chinese.
-
Long-term follow-up after catheter ablation of paroxysmal atrial fibrillation: the incidence of recurrence and progression of atrial fibrillation.Circ Arrhythm Electrophysiol. 2014 Apr;7(2):267-73. doi: 10.1161/CIRCEP.113.000471. Epub 2014 Mar 7. Circ Arrhythm Electrophysiol. 2014. PMID: 24610740
-
Safety and efficacy of early radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation complicated with amiodarone-induced thyrotoxicosis.Cardiol J. 2016;23(4):416-21. doi: 10.5603/CJ.a2016.0029. Epub 2016 Jun 14. Cardiol J. 2016. PMID: 27296156
-
Catheter Ablation for Long-Standing Persistent Atrial Fibrillation.Methodist Debakey Cardiovasc J. 2015 Apr-Jun;11(2):87-93. doi: 10.14797/mdcj-11-2-87. Methodist Debakey Cardiovasc J. 2015. PMID: 26306125 Free PMC article. Review.
-
Catheter Ablation of Paroxysmal Atrial Fibrillation: Have We Achieved Cure with Pulmonary Vein Isolation?Methodist Debakey Cardiovasc J. 2015 Apr-Jun;11(2):71-5. doi: 10.14797/mdcj-11-2-71. Methodist Debakey Cardiovasc J. 2015. PMID: 26306122 Free PMC article. Review.
Cited by
-
Common variants for atrial fibrillation: results from genome-wide association studies.Hum Genet. 2012 Jan;131(1):33-9. doi: 10.1007/s00439-011-1052-3. Epub 2011 Jul 7. Hum Genet. 2012. PMID: 21735173 Review.
-
Comparison of Atrial Fibrillation in the Young versus That in the Elderly: A Review.Cardiol Res Pract. 2013;2013:976976. doi: 10.1155/2013/976976. Epub 2013 Jan 22. Cardiol Res Pract. 2013. PMID: 23401843 Free PMC article.
-
Functional characterization of ABCC9 variants identified in sudden unexpected natural death.Forensic Sci Int. 2019 May;298:80-87. doi: 10.1016/j.forsciint.2019.02.035. Epub 2019 Feb 27. Forensic Sci Int. 2019. PMID: 30878466 Free PMC article.
-
Contemporary management of atrial fibrillation: update on anticoagulation and invasive management strategies.Mayo Clin Proc. 2009 Jul;84(7):643-62. doi: 10.1016/S0025-6196(11)60754-4. Mayo Clin Proc. 2009. PMID: 19567719 Free PMC article. Review.
-
Gain of function in IKs secondary to a mutation in KCNE5 associated with atrial fibrillation.Heart Rhythm. 2008 Mar;5(3):427-35. doi: 10.1016/j.hrthm.2007.12.019. Epub 2008 Feb 4. Heart Rhythm. 2008. PMID: 18313602 Free PMC article.
References
-
- Olson TM, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. - PubMed
-
- Matsushita K, et al. Intramolecular interaction of SUR2 subtypes for intracellular ADP-Induced differential control of KATP channels. Circ Res. 2002;90:554–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
