Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features
- PMID: 15583126
- PMCID: PMC536014
- DOI: 10.1073/pnas.0404756101
Molecular basis of Kir6.2 mutations associated with neonatal diabetes or neonatal diabetes plus neurological features
Abstract
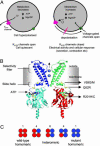
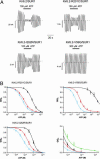
Inwardly rectifying potassium channels (Kir channels) control cell membrane K(+) fluxes and electrical signaling in diverse cell types. Heterozygous mutations in the human Kir6.2 gene (KCNJ11), the pore-forming subunit of the ATP-sensitive (K(ATP)) channel, cause permanent neonatal diabetes mellitus (PNDM). For some mutations, PNDM is accompanied by marked developmental delay, muscle weakness, and epilepsy (severe disease). To determine the molecular basis of these different phenotypes, we expressed wild-type or mutant (R201C, Q52R, or V59G) Kir6.2/sulfonylurea receptor 1 channels in Xenopus oocytes. All mutations increased resting whole-cell K(ATP) currents by reducing channel inhibition by ATP, but, in the simulated heterozygous state, mutations causing PNDM alone (R201C) produced smaller K(ATP) currents and less change in ATP sensitivity than mutations associated with severe disease (Q52R and V59G). This finding suggests that increased K(ATP) currents hyperpolarize pancreatic beta cells and impair insulin secretion, whereas larger K(ATP) currents are required to influence extrapancreatic cell function. We found that mutations causing PNDM alone impair ATP sensitivity directly (at the binding site), whereas those associated with severe disease act indirectly by biasing the channel conformation toward the open state. The effect of the mutation on ATP sensitivity in the heterozygous state reflects the different contributions of a single subunit in the Kir6.2 tetramer to ATP inhibition and to the energy of the open state. Our results also show that mutations in the slide helix of Kir6.2 (V59G) influence the channel kinetics, providing evidence that this domain is involved in Kir channel gating, and suggest that the efficacy of sulfonylurea therapy in PNDM may vary with genotype.
Figures


Similar articles
-
Kir6.2 mutations associated with neonatal diabetes reduce expression of ATP-sensitive K+ channels: implications in disease mechanism and sulfonylurea therapy.Diabetes. 2006 Jun;55(6):1738-46. doi: 10.2337/db05-1571. Diabetes. 2006. PMID: 16731837
-
Mutations at the same residue (R50) of Kir6.2 (KCNJ11) that cause neonatal diabetes produce different functional effects.Diabetes. 2006 Jun;55(6):1705-12. doi: 10.2337/db05-1640. Diabetes. 2006. PMID: 16731833
-
Functional analysis of six Kir6.2 (KCNJ11) mutations causing neonatal diabetes.Pflugers Arch. 2006 Dec;453(3):323-32. doi: 10.1007/s00424-006-0112-3. Epub 2006 Sep 22. Pflugers Arch. 2006. PMID: 17021801
-
Diabetes and hypoglycaemia in young children and mutations in the Kir6.2 subunit of the potassium channel: therapeutic consequences.Diabetes Metab. 2006 Dec;32(6):569-80. doi: 10.1016/S1262-3636(07)70311-7. Diabetes Metab. 2006. PMID: 17296510 Review.
-
Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11.Rev Endocr Metab Disord. 2010 Sep;11(3):193-8. doi: 10.1007/s11154-010-9149-x. Rev Endocr Metab Disord. 2010. PMID: 20922570 Review.
Cited by
-
Molecular mechanism of sulphonylurea block of K(ATP) channels carrying mutations that impair ATP inhibition and cause neonatal diabetes.Diabetes. 2013 Nov;62(11):3909-19. doi: 10.2337/db13-0531. Epub 2013 Jul 8. Diabetes. 2013. PMID: 23835339 Free PMC article.
-
Functional characterization of a novel KCNJ11 in frame mutation-deletion associated with infancy-onset diabetes and a mild form of intermediate DEND: a battle between K(ATP) gain of channel activity and loss of channel expression.PLoS One. 2013 May 7;8(5):e63758. doi: 10.1371/journal.pone.0063758. Print 2013. PLoS One. 2013. PMID: 23667671 Free PMC article.
-
A gating mutation at the internal mouth of the Kir6.2 pore is associated with DEND syndrome.EMBO Rep. 2005 May;6(5):470-5. doi: 10.1038/sj.embor.7400393. EMBO Rep. 2005. PMID: 15864298 Free PMC article.
-
Sulfonylurea therapy in two Korean patients with insulin-treated neonatal diabetes due to heterozygous mutations of the KCNJ11 gene encoding Kir6.2.J Korean Med Sci. 2007 Aug;22(4):616-20. doi: 10.3346/jkms.2007.22.4.616. J Korean Med Sci. 2007. PMID: 17728498 Free PMC article.
-
Prevalence of permanent neonatal diabetes in Slovakia and successful replacement of insulin with sulfonylurea therapy in KCNJ11 and ABCC8 mutation carriers.J Clin Endocrinol Metab. 2007 Apr;92(4):1276-82. doi: 10.1210/jc.2006-2490. Epub 2007 Jan 9. J Clin Endocrinol Metab. 2007. PMID: 17213273 Free PMC article.
References
-
- Seino, S. & Miki, T. (2003) Prog. Biophys. Mol. Biol. 81, 133–176. - PubMed
-
- Tucker, S. J., Gribble, F. M., Zhao, C., Trapp, S. & Ashcroft, F. M. (1997) Nature 387, 179–183. - PubMed
-
- Gloyn, A. L., Pearson, E. R., Antcliff, J. F., Proks, P., Bruining, G. J., Slingerland, A. S., Howard, N., Srinivasan, S., Silva, J. M., Molnes, J., et al. (2004) N. Engl. J. Med. 350, 1838–1849. - PubMed
-
- Vaxillaire, M., Populaire, C., Busiah, K., Cave, H., Gloyn, A. L., Hattersley, A. T., Czernichow, P., Froguel, P. & Polak, M. (2004) Diabetes 53, 2719–2722. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

