Subcutaneous efgartigimod PH20 in generalized myasthenia gravis: A phase 3 randomized noninferiority study (ADAPT-SC) and interim analyses of a long-term open-label extension study (ADAPT-SC+)
- PMID: 39227284
- PMCID: PMC11579873
- DOI: 10.1016/j.neurot.2024.e00378
Subcutaneous efgartigimod PH20 in generalized myasthenia gravis: A phase 3 randomized noninferiority study (ADAPT-SC) and interim analyses of a long-term open-label extension study (ADAPT-SC+)
Abstract
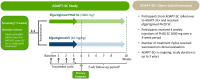
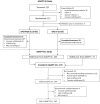
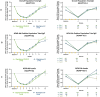
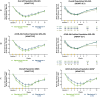
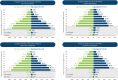
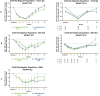
ADAPT-SC (NCT04735432) was designed to evaluate noninferiority of subcutaneous (SC) efgartigimod PH20 to intravenous (IV) efgartigimod in participants with generalized myasthenia gravis (gMG). ADAPT-SC+ (NCT04818671) is an open-label extension study designed to assess long-term safety, tolerability, and efficacy of efgartigimod PH20 SC. Adult participants in ADAPT-SC were randomly assigned to receive a treatment cycle of 4 once-weekly administrations of efgartigimod PH20 SC 1000 mg or efgartigimod IV 10 mg/kg, followed by 7 weeks of follow-up. Primary endpoint was percentage change from baseline in total immunoglobulin G (IgG) level at week 4 (1 week after the fourth administration). Secondary efficacy endpoints assessed number and percentage of Myasthenia Gravis Activities of Daily Living (MG-ADL) and Quantitative Myasthenia Gravis (QMG) responders and mean change from baseline in total score for each measure. The primary endpoint was met, demonstrating noninferiority in total IgG reduction between efgartigimod PH20 SC 1000 mg and efgartigimod IV 10 mg/kg. Clinically meaningful improvements were seen as early as 1 week following the first administration in both treatment arms, with maximal improvements at week 4. Continued treatment cycles of efgartigimod PH20 SC in ADAPT-SC+ have demonstrated long-term safety and consistent improvements in MG-ADL total score. Findings from ADAPT-SC and ADAPT-SC+ demonstrate similar safety and efficacy as observed in the placebo-controlled ADAPT study. Collectively, these findings support noninferiority between efgartigimod PH20 SC 1000 mg and efgartigimod IV 10 mg/kg, as well as long-term safety, tolerability, and efficacy of efgartigimod PH20 SC for treatment of a broad population of patients with gMG.
Keywords: Efgartigimod; FcRn; IgG recycling; Myasthenia gravis; Neonatal Fc receptor antagonist.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest This study was sponsored by argenx (Ghent, Belgium), the manufacturer of efgartigimod IV and efgartigimod PH20 SC. Efgartigimod IV has received regulatory approval for the treatment of gMG in multiple countries. Efgartigimod PH20 SC was approved for use in gMG by the US Food and Drug Administration. Medical writing and editorial support were funded by argenx. Li Liu, Fien Gistelinck, Sophie Steeland, Benjamin Van Hoorick, Jana Podhorna, and Filip Borgions are employees of argenx. Jan Noukens is partner at Curare Consulting and is a paid consultant for argenx. James F. Howard, Jr has received research support (paid to his institution) from Alexion Pharmaceuticals, argenx, Cartesian Therapeutics, the US Centers for Disease Control and Prevention, the Myasthenia Gravis Foundation of America, the Muscular Dystrophy Association, the US National Institutes of Health (including the National Institute of Neurological Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Diseases), Patient-Centered Outcomes Research Institute, Ra Pharmaceuticals (now UCB Biosciences), and Millennium Pharmaceuticals/Takeda Pharmaceuticals; honoraria from AcademicCME, Alexion Pharmaceuticals, argenx BV, Biologix Pharma, F. Hoffman-LaRoche Ltd, Horizon Therapeutics plc, Merck EMD Serono, NMD Pharma, Novartis Pharmaceuticals, PeerView CME, Ra Pharmaceuticals (now UCB Biosciences), Regeneron Pharmaceuticals, and Sanofi US; and non-financial support from Alexion Pharmaceuticals, argenx BV, Ra Pharmaceuticals (now UCB Biosciences), Toleranzia AB, and ZaiLab. Tuan Vu serves as site principal investigator for MG clinical trials sponsored by argenx, Alexion, UCB/Ra, Cartesian, Horizon/Viela Bio, Janssen/Momenta, Regeneron, Immunovant, and Sanofi and has served as consultant and/or speaker for argenx, Alexion, and UCB/Ra. George Li has nothing to disclose. Denis Korobko has received speaker honoraria from Roche-Moscow, Novartis Russia, Sanofi, Merck, Janssen (Johnson & Johnson company), BIOCAD; research grants from Novartis Russia, UCB, argenx, Viela Bio Inc. (now Horizon Therapeutics), Sanofi, Bristol Myers Squibb, Hoffman-LaRoche Ltd.; and has served on scientific advisory boards for Novartis Russia, Merck, Janssen (Johnson & Johnson company), and BIOCAD. Marek Smilowski has nothing to report. Yuebing Li has served on advisory boards for argenx, Catalyst, Immunovant, and UCB Pharma and has received grant support from argenx. Kimiaki Utsugisawa has served as a paid consultant for argenx, UCB Pharma, Janssen Pharma, Viela Bio, Chugai Pharma, Merck, and Mitsubishi Tanabe Pharma and has received speaker honoraria from argenx, Alexion Pharmaceuticals, UCB Pharma, and the Japan Blood Products Organization. Heinz Wiendl receives honoraria for acting as a member of scientific advisory boards for AbbVie, Alexion, argenx, Bristol Myers Squibb, Janssen, Merck, Novartis, and Sandoz; has received speaker honoraria and travel support from Alexion, Biogen, Bristol Myers Squibb, Genzyme, Merck, Neurodiem, Novartis, Ology, Roche, Teva, and WebMD Global; has received research funding from Deutsche Forschungsgesellschaft (DFG), Deutsche Myasthenie Gesellschaft e.V., European Union, Alexion, Amicus Therapeutics, argenx, Biogen, CSL Behring, F. Hoffmann-La Roche, Genzyme, Merck KgaA, Novartis, Roche Pharma, and UCB Biopharma; and is a paid consultant for AbbVie, Actelion, argenx, BD, Bristol Myers Squibb, EMD Serono, Fondazione Cariplo, Gossamer Bio, Idorsia, Immunic, Immunovant, INmune Bio, Syneos Health, Janssen, Merck, NexGen, Novartis, Roche, Sanofi, Swiss Multiple Sclerosis Society, UCB, and Worldwide Clinical Trials. Jan L. De Bleecker has served as a paid consultant for or received speaker honoraria from argenx, UCB Pharma, Alexion Pharmaceuticals, Sanofi, CSL Behring, and Roche. Renato Mantegazza has received funding for travel, meeting attendance, or advisory board participation from Alexion, argenx, Biomarin, Catalyst, Sanofi, Regeneron, and UCB.
Figures
Similar articles
-
Long-term safety, tolerability, and efficacy of efgartigimod (ADAPT+): interim results from a phase 3 open-label extension study in participants with generalized myasthenia gravis.Front Neurol. 2024 Jan 17;14:1284444. doi: 10.3389/fneur.2023.1284444. eCollection 2023. Front Neurol. 2024. PMID: 38318236 Free PMC article.
-
Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial.Lancet Neurol. 2021 Jul;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9. Lancet Neurol. 2021. PMID: 34146511 Clinical Trial.
-
Effect of efgartigimod on muscle group subdomains in participants with generalized myasthenia gravis: post hoc analyses of the phase 3 pivotal ADAPT study.Eur J Neurol. 2024 Jan;31(1):e16098. doi: 10.1111/ene.16098. Epub 2023 Oct 16. Eur J Neurol. 2024. PMID: 37843174 Free PMC article.
-
Clinical efficacy and safety of efgartigimod for treatment of myasthenia gravis.Immunotherapy. 2023 Jun;15(8):553-563. doi: 10.2217/imt-2022-0298. Epub 2023 Apr 4. Immunotherapy. 2023. PMID: 37013835 Review.
-
The efficacy and safety of FcRn inhibitors in patients with myasthenia gravis: a systematic review and meta-analysis.J Neurol. 2024 May;271(5):2298-2308. doi: 10.1007/s00415-024-12247-x. Epub 2024 Mar 3. J Neurol. 2024. PMID: 38431900 Review.
Cited by
-
Efgartigimod: A Review in Generalised Myasthenia Gravis.Drugs. 2024 Nov;84(11):1463-1474. doi: 10.1007/s40265-024-02101-9. Epub 2024 Nov 7. Drugs. 2024. PMID: 39511131 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

