Drug-induced liver injury associated with atypical generation antipsychotics from the FDA Adverse Event Reporting System (FAERS)
- PMID: 39215339
- PMCID: PMC11363531
- DOI: 10.1186/s40360-024-00782-2
Drug-induced liver injury associated with atypical generation antipsychotics from the FDA Adverse Event Reporting System (FAERS)
Abstract
Background: Recent studies have shown that liver enzyme abnormalities were not only seen with typical antipsychotics (APs) but also with atypical antipsychotics (AAPs). During the last 20 years, the hepatotoxicity of various antipsychotics received much attention. However, systematic evaluations of hepatotoxicity associated with APs are limited.
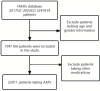
Methods: All drug related hepatic disorders cases were retrieved from the FDA Adverse Event Reporting System (FAERS) database using standardized MedDRA queries (SMQ) from the first quarter of 2017 to the first quarter of 2022. Patient characteristics and prognosis were assessed. In this study, a case/non-case approach was used to calculate reporting odds ratio (RORs) and 95% confidence intervals (CIs). We calculated the drug-induced liver injury (DILI) RORs for each AAPs.
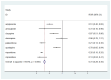
Results: A total of 408 DILI cases were attributed to AAPs during the study period. 18.6% of these were designated as serious adverse event (SAE), which include death (19.74%), hospitalization (68.42%), disability (2.63%), and life-threatening (9.21%) outcomes. The RORs values in descending order were: quetiapine (ROR = 0.782), clozapine (ROR = 0.665), aripiprazole (ROR = 0.507), amisulpride (ROR = 0.308), paliperidone (ROR = 0.212), risperidone (ROR = 0.198), ziprasidone (0.131).
Conclusion: The result found in our study was that all AAPs didn't have a significant correlation with increased hepatotoxicity. Future analysis of the FAERS database in conjunction with other data sources will be essential for continuous monitoring of DILI.
Keywords: AAPs; DILI; FAERS; Hepatotoxicity; Reporting odds ratio (RORs); SAE.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Comparative Safety Signal Assessment of Hospitalization Associated With the Use of Atypical Antipsychotics.Front Psychiatry. 2022 Jun 6;13:917351. doi: 10.3389/fpsyt.2022.917351. eCollection 2022. Front Psychiatry. 2022. PMID: 35733796 Free PMC article.
-
Drug-induced liver injury associated with antiseizure medications from the FDA Adverse Event Reporting System (FAERS).Epilepsy Behav. 2021 Apr;117:107832. doi: 10.1016/j.yebeh.2021.107832. Epub 2021 Feb 21. Epilepsy Behav. 2021. PMID: 33626490
-
Drug-induced liver injury associated with elexacaftor/tezacaftor/ivacaftor: A pharmacovigilance analysis of the FDA adverse event reporting system (FAERS).J Cyst Fibros. 2024 May;23(3):566-572. doi: 10.1016/j.jcf.2024.01.001. Epub 2024 Jan 16. J Cyst Fibros. 2024. PMID: 38233246
-
Summary of the comparative effectiveness review on off-label use of atypical antipsychotics.J Manag Care Pharm. 2012 Jun;18(5 Suppl B):S1-20. doi: 10.18553/jmcp.2012.18.s5-b.1. J Manag Care Pharm. 2012. PMID: 22784311 Free PMC article. Review.
-
An open, large, 6-month naturalistic study of outcome in schizophrenic outpatients, treated with olanzapine.Hum Psychopharmacol. 2011 Jan;26(1):81-5. doi: 10.1002/hup.1173. Epub 2011 Feb 9. Hum Psychopharmacol. 2011. PMID: 23055416 Review.
Cited by
-
Pharmacovigilance in Action: Utilizing VigiBase Data to Improve Clozapine Safety.Patient Prefer Adherence. 2024 Nov 12;18:2261-2280. doi: 10.2147/PPA.S495254. eCollection 2024. Patient Prefer Adherence. 2024. PMID: 39553897 Free PMC article. Review.
References
-
- Findling RL, Steiner H, Weller EB. Use of antipsychotics in children and adolescents. J Clin Psychiatry. 2005;66(Suppl 7):29–40. - PubMed
-
- Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral and long-acting injectable antipsychotics for the maintenance treatment of adults with schizophrenia: a systematic review and network meta-analysis. Lancet. 2022;399(10327):824–36. 10.1016/S0140-6736(21)01997-8 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous