Poor reporting quality of randomized controlled trials comparing treatments of COVID-19-A retrospective cross-sectional study on the first year of publications
- PMID: 37844082
- PMCID: PMC10578566
- DOI: 10.1371/journal.pone.0292860
Poor reporting quality of randomized controlled trials comparing treatments of COVID-19-A retrospective cross-sectional study on the first year of publications
Abstract
Introduction: Transparent and complete reporting of randomized controlled trials (RCTs) is essential for critical scientific appraisal of the results. It has been argued whether publications during the COVID-19 pandemic have met reporting standards. In this study, we assessed reporting adherence of RCTs on treatment interventions in COVID-19 patients to the CONSORT checklist and discuss which lessons can be learned to improve reporting in the future.
Methods: This was a retrospective, cross-sectional study performed at the University Hospital RWTH Aachen, Germany. We conducted a pragmatic systematic literature search in the PubMed database to identify RCTs on treatment interventions in COVID-19 patients in the first year of publications on the topic (March 2020-February 2021). We investigated the adherence of each publication to the CONSORT checklist and assessed the association between specific predictors and percentage adherence in an exploratory multivariable regression model.
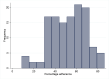
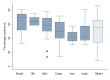
Results: We analyzed 127 RCTs and found that the median percentage adherence to the CONSORT checklist was 54.3% [IQR 38.9 to 65.7]. In the exploratory multivariable regression model, the impact factor (highest tertile of impact factor compared to lowest tertile ß = 21.77, 95% CI 13.89 to 29.66, p<0.001; middle tertile compared lowest tertile ß = 11.79, 95% CI 5.74 to 17.84, p<0.001)) and authors' referral to the CONSORT statement (ß = 9.29, 95% CI 2.98 to 15.60, p = 0.004) were associated with a higher percentage adherence to the CONSORT checklist.
Conclusion: The reporting quality of RCTs on treatment interventions in COVID-19 patients during the first year of publications was poor. Measures to improve reporting quality are urgently needed.
Copyright: © 2023 Grüßer et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article. Review.
-
Evaluation of reporting quality of randomized controlled trials in patients with COVID-19 using the CONSORT statement.PLoS One. 2021 Sep 23;16(9):e0257093. doi: 10.1371/journal.pone.0257093. eCollection 2021. PLoS One. 2021. PMID: 34555033 Free PMC article.
-
Reporting Quality of Randomized Controlled Trials of Periodontal Diseases in Journal Abstracts-A Cross-sectional Survey and Bibliometric Analysis.J Evid Based Dent Pract. 2018 Jun;18(2):130-141.e22. doi: 10.1016/j.jebdp.2017.08.005. Epub 2017 Sep 21. J Evid Based Dent Pract. 2018. PMID: 29747793
-
Poor reporting quality of observational clinical studies comparing treatments of COVID-19 - a retrospective cross-sectional study.BMC Med Res Methodol. 2022 Jan 20;22(1):23. doi: 10.1186/s12874-021-01501-9. BMC Med Res Methodol. 2022. PMID: 35057739 Free PMC article.
-
Using the CONSORT statement to evaluate the completeness of reporting of addiction randomised trials: a cross-sectional review.BMJ Open. 2019 Sep 6;9(9):e032024. doi: 10.1136/bmjopen-2019-032024. BMJ Open. 2019. PMID: 31494625 Free PMC article. Review.
References
-
- McGovern DPB, Valori RM, Summerskill WSM, Levi M. Key Topics in Evidence-Based Medicine: BIOS Scientific Publishers; 2001.
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al.. Improving the Quality of Reporting of Randomized Controlled Trials: The CONSORT Statement. JAMA. 1996;276(8):637–9. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous