Pegcetacoplan for Treating Paroxysmal Nocturnal Haemoglobinuria: An Evidence Review Group Perspective of a NICE Single Technology Appraisal
- PMID: 37195551
- PMCID: PMC10333166
- DOI: 10.1007/s41669-023-00408-z
Pegcetacoplan for Treating Paroxysmal Nocturnal Haemoglobinuria: An Evidence Review Group Perspective of a NICE Single Technology Appraisal
Abstract
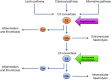
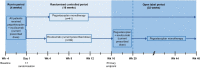
As part of the Single Technology Appraisal (STA) process, the UK National Institute for Health and Care Excellence (NICE) invited Apellis Pharmaceuticals/Sobi to submit evidence for the clinical and cost effectiveness of pegcetacoplan versus eculizumab and pegcetacoplan versus ravulizumab for treating paroxysmal nocturnal haemoglobinuria (PNH) in adults whose anaemia is uncontrolled after treatment with a C5 inhibitor. The Liverpool Reviews and Implementation Group at the University of Liverpool was commissioned as the Evidence Review Group (ERG). The company pursued a low incremental cost-effectiveness ratio (ICER) Fast Track Appraisal (FTA). This was a form of STA processed in a shorter time frame and designed for technologies with company base-case ICER < £10,000 per quality-adjusted life-year (QALY) gained and most plausible ICER < £20,000 per QALY gained. This article summarises the ERG's review of the company's evidence submission, and the NICE Appraisal Committee's (AC's) final decision. The company presented clinical evidence from the PEGASUS trial that assessed the efficacy of pegcetacoplan versus eculizumab. At Week 16, patients in the pegcetacoplan arm had statistically significantly greater change from baseline in haemoglobin levels and a higher rate of transfusion avoidance than patients in the eculizumab arm. Using the PEGASUS trial and Study 302 data (a non-inferiority trial that assessed ravulizumab versus eculizumab), the company conducted an anchored matching-adjusted indirect comparison (MAIC) to indirectly estimate the efficacy of pegcetacoplan versus ravulizumab. The company identified key differences between trial designs and populations that could not be adjusted for using anchored MAIC methods. The company and ERG agreed that the anchored MAIC results were not robust and should not inform decision making. In the absence of robust indirect estimates, the company assumed that ravulizumab had equivalent efficacy to eculizumab in the PEGASUS trial population. Results from the company base-case cost-effectiveness analysis showed that treatment with pegcetacoplan dominated eculizumab and ravulizumab. The ERG considered that the long-term effectiveness of pegcetacoplan was uncertain and ran a scenario assuming that after 1 year the efficacy of pegcetacoplan would be the same as eculizumab; treatment with pegcetacoplan continued to dominate eculizumab and ravulizumab. The AC noted that treatment with pegcetacoplan had lower total costs than treatment with eculizumab or ravulizumab because it is self-administered and reduces the need for blood transfusions. If the assumption that ravulizumab has equivalent efficacy to eculizumab does not hold, then this will affect the estimate of the cost effectiveness of pegcetacoplan versus ravulizumab; however, the AC was satisfied that the assumption was reasonable. The AC recommended pegcetacoplan as an option for the treatment of PNH in adults who have uncontrolled anaemia despite treatment with a stable dose of a C5 inhibitor for ≥ 3 months. Pegcetacoplan was the first technology recommended by NICE via the low ICER FTA process.
© 2023. The Author(s).
Conflict of interest statement
The authors have no competing interests. This article was not externally peer reviewed by
Figures
Similar articles
-
Pegylated Liposomal Irinotecan Hydrochloride Trihydrate for Treating Pancreatic Cancer After Gemcitabine: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Mar;36(3):289-299. doi: 10.1007/s40273-017-0592-3. Pharmacoeconomics. 2018. PMID: 29178025 Review.
-
Comparative effectiveness of pegcetacoplan versus ravulizumab in patients with paroxysmal nocturnal hemoglobinuria previously treated with eculizumab: a matching-adjusted indirect comparison.Curr Med Res Opin. 2021 Nov;37(11):1913-1923. doi: 10.1080/03007995.2021.1971182. Epub 2021 Sep 3. Curr Med Res Opin. 2021. PMID: 34445916
-
Dinutuximab Beta for Treating Neuroblastoma: An Evidence Review Group and Decision Support Unit Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2019 Aug;37(8):985-993. doi: 10.1007/s40273-018-0744-0. Pharmacoeconomics. 2019. PMID: 30465228 Review.
-
Venetoclax for Treating Chronic Lymphocytic Leukaemia: An Evidence Review Group Perspective of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Apr;36(4):399-406. doi: 10.1007/s40273-017-0599-9. Pharmacoeconomics. 2018. PMID: 29222670 Free PMC article. Review.
-
Etelcalcetide for Treating Secondary Hyperparathyroidism: An Evidence Review Group Evaluation of a NICE Single Technology Appraisal.Pharmacoeconomics. 2018 Nov;36(11):1299-1308. doi: 10.1007/s40273-018-0661-2. Pharmacoeconomics. 2018. PMID: 29691773 Review.
References
-
- National Institute for Health and Care Excellence. Technology appraisal processes. https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/ni.... Accessed 24 June 2022.
-
- National Institute for Health and Care Excellence. Pegcetacoplan for treating paroxysmal nocturnal haemoglobinuria [ID3746]. https://www.nice.org.uk/guidance/indevelopment/gid-ta10651/documents. Accessed 7 June 2022.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

