Opioids and Vitamin C: Known Interactions and Potential for Redox-Signaling Crosstalk
- PMID: 35883757
- PMCID: PMC9312198
- DOI: 10.3390/antiox11071267
Opioids and Vitamin C: Known Interactions and Potential for Redox-Signaling Crosstalk
Abstract
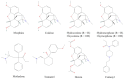
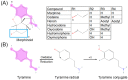
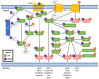
Opioids are among the most widely used classes of pharmacologically active compounds both clinically and recreationally. Beyond their analgesic efficacy via μ opioid receptor (MOR) agonism, a prominent side effect is central respiratory depression, leading to systemic hypoxia and free radical generation. Vitamin C (ascorbic acid; AA) is an essential antioxidant vitamin and is involved in the recycling of redox cofactors associated with inflammation. While AA has been shown to reduce some of the negative side effects of opioids, the underlying mechanisms have not been explored. The present review seeks to provide a signaling framework under which MOR activation and AA may interact. AA can directly quench reactive oxygen and nitrogen species induced by opioids, yet this activity alone does not sufficiently describe observations. Downstream of MOR activation, confounding effects from AA with STAT3, HIF1α, and NF-κB have the potential to block production of antioxidant proteins such as nitric oxide synthase and superoxide dismutase. Further mechanistic research is necessary to understand the underlying signaling crosstalk of MOR activation and AA in the amelioration of the negative, potentially fatal side effects of opioids.
Keywords: crosstalk; metabolism; mu opioid receptor; opioids; oxidative stress; signaling; vitamin C.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Nitric oxide and zinc-mediated protein assemblies involved in mu opioid receptor signaling.Mol Neurobiol. 2013 Dec;48(3):769-82. doi: 10.1007/s12035-013-8465-z. Epub 2013 May 11. Mol Neurobiol. 2013. PMID: 23666425 Review.
-
Influence of vitamin C and vitamin E on redox signaling: Implications for exercise adaptations.Free Radic Biol Med. 2015 Jul;84:65-76. doi: 10.1016/j.freeradbiomed.2015.03.018. Epub 2015 Apr 2. Free Radic Biol Med. 2015. PMID: 25841784 Review.
-
Spinal Opioid Tolerance Depends upon Platelet-Derived Growth Factor Receptor-β Signaling, Not μ-Opioid Receptor Internalization.Mol Pharmacol. 2020 Oct;98(4):487-496. doi: 10.1124/mol.120.119552. Epub 2020 Jul 28. Mol Pharmacol. 2020. PMID: 32723769 Free PMC article.
-
Assessment of structure-activity relationships and biased agonism at the Mu opioid receptor of novel synthetic opioids using a novel, stable bio-assay platform.Biochem Pharmacol. 2020 Jul;177:113910. doi: 10.1016/j.bcp.2020.113910. Epub 2020 Mar 14. Biochem Pharmacol. 2020. PMID: 32179045
-
The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress.Free Radic Biol Med. 2013 Dec;65:1174-1194. doi: 10.1016/j.freeradbiomed.2013.09.001. Epub 2013 Sep 12. Free Radic Biol Med. 2013. PMID: 24036104 Review.
Cited by
-
Impact of vitamin C on the reduction of opioid consumption after an emergency department visit for acute musculoskeletal pain: a double-blind randomised control trial protocol.BMJ Open. 2023 May 24;13(5):e069230. doi: 10.1136/bmjopen-2022-069230. BMJ Open. 2023. PMID: 37225265 Free PMC article.
-
Higher serum ascorbic acid levels are associated with lower depression prevalence in US adults: a case-control study.Front Nutr. 2024 Jan 26;11:1324835. doi: 10.3389/fnut.2024.1324835. eCollection 2024. Front Nutr. 2024. PMID: 38344022 Free PMC article.
-
Opioid Therapy and Implications for Oxidative Balance: A Clinical Study of Total Oxidative Capacity (TOC) and Total Antioxidative Capacity (TAC).J Clin Med. 2023 Dec 22;13(1):82. doi: 10.3390/jcm13010082. J Clin Med. 2023. PMID: 38202088 Free PMC article.
-
Fentanyl Overdose Causes Prolonged Cardiopulmonary Dysregulation in Male SKH1 Mice.Pharmaceuticals (Basel). 2024 Jul 14;17(7):941. doi: 10.3390/ph17070941. Pharmaceuticals (Basel). 2024. PMID: 39065791 Free PMC article.
-
The cell-permeant antioxidant D-thiol ester D-cysteine ethyl ester overcomes physical dependence to morphine in male Sprague Dawley rats.Front Pharmacol. 2024 Aug 26;15:1444574. doi: 10.3389/fphar.2024.1444574. eCollection 2024. Front Pharmacol. 2024. PMID: 39253377 Free PMC article.
References
-
- Swegle J.M., Logemann C. Management of common opioid-induced adverse effects. Am. Fam. Physician. 2006;74:1347–1354. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

