Antiviral Activity of the Rhamnolipids Mixture from the Antarctic Bacterium Pseudomonas gessardii M15 against Herpes Simplex Viruses and Coronaviruses
- PMID: 34959400
- PMCID: PMC8704987
- DOI: 10.3390/pharmaceutics13122121
Antiviral Activity of the Rhamnolipids Mixture from the Antarctic Bacterium Pseudomonas gessardii M15 against Herpes Simplex Viruses and Coronaviruses
Abstract
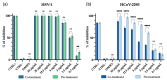
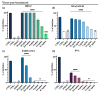
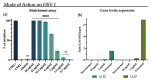
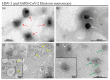
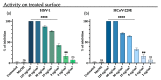
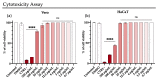
Emerging and re-emerging viruses represent a serious threat to human health at a global level. In particular, enveloped viruses are one of the main causes of viral outbreaks, as recently demonstrated by SARS-CoV-2. An effective strategy to counteract these viruses could be to target the envelope by using surface-active compounds. Rhamnolipids (RLs) are microbial biosurfactants displaying a wide range of bioactivities, such as antibacterial, antifungal and antibiofilm, among others. Being of microbial origin, they are environmentally-friendly, biodegradable, and less toxic than synthetic surfactants. In this work, we explored the antiviral activity of the rhamnolipids mixture (M15RL) produced by the Antarctic bacteria Pseudomonas gessardii M15 against viruses belonging to Coronaviridae and Herpesviridae families. In addition, we investigated the rhamnolipids' mode of action and the possibility of inactivating viruses on treated surfaces. Our results show complete inactivation of HSV-1 and HSV-2 by M15RLs at 6 µg/mL, and of HCoV-229E and SARS-CoV-2 at 25 and 50 µg/mL, respectively. Concerning activity against HCoV-OC43, 80% inhibition of cytopathic effect was recorded, while no activity against naked Poliovirus Type 1 (PV-1) was detectable, suggesting that the antiviral action is mainly directed towards the envelope. In conclusion, we report a significant activity of M15RL against enveloped viruses and demonstrated for the first time the antiviral effect of rhamnolipids against SARS-CoV-2.
Keywords: Antarctic bacteria; SARS-CoV-2; TEM; antiviral; coronavirus; enveloped virus; herpes; microbial biosurfactants; rhamnolipids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Evaluation of Antimicrobial Properties and Potential Applications of Pseudomonas gessardii M15 Rhamnolipids towards Multiresistant Staphylococcus aureus.Pharmaceutics. 2023 Feb 19;15(2):700. doi: 10.3390/pharmaceutics15020700. Pharmaceutics. 2023. PMID: 36840022 Free PMC article.
-
Antiviral Activity of Ficus rubiginosa Leaf Extracts against HSV-1, HCoV-229E and PV-1.Viruses. 2022 Oct 14;14(10):2257. doi: 10.3390/v14102257. Viruses. 2022. PMID: 36298811 Free PMC article.
-
Application of Environment-Friendly Rhamnolipids against Transmission of Enveloped Viruses Like SARS-CoV2.Viruses. 2021 Feb 20;13(2):322. doi: 10.3390/v13020322. Viruses. 2021. PMID: 33672561 Free PMC article.
-
Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies.Viruses. 2012 Nov 12;4(11):3044-68. doi: 10.3390/v4113044. Viruses. 2012. PMID: 23202515 Free PMC article. Review.
-
Properties of Coronavirus and SARS-CoV-2.Malays J Pathol. 2020 Apr;42(1):3-11. Malays J Pathol. 2020. PMID: 32342926 Review.
Cited by
-
The Broad-Spectrum Antiviral Potential of the Amphibian Peptide AR-23.Int J Mol Sci. 2022 Jan 14;23(2):883. doi: 10.3390/ijms23020883. Int J Mol Sci. 2022. PMID: 35055066 Free PMC article.
-
New Imidazolium Alkaloids with Broad Spectrum of Action from the Marine Bacterium Shewanella aquimarina.Pharmaceutics. 2023 Aug 14;15(8):2139. doi: 10.3390/pharmaceutics15082139. Pharmaceutics. 2023. PMID: 37631353 Free PMC article.
-
Antiviral Properties of Moringa oleifera Leaf Extracts against Respiratory Viruses.Viruses. 2024 Jul 25;16(8):1199. doi: 10.3390/v16081199. Viruses. 2024. PMID: 39205173 Free PMC article.
-
Cryosphere: a frozen home of microbes and a potential source for drug discovery.Arch Microbiol. 2024 Mar 28;206(4):196. doi: 10.1007/s00203-024-03899-4. Arch Microbiol. 2024. PMID: 38546887 Review.
-
The First Pseudomonas Phage vB_PseuGesM_254 Active against Proteolytic Pseudomonas gessardii Strains.Viruses. 2024 Sep 30;16(10):1561. doi: 10.3390/v16101561. Viruses. 2024. PMID: 39459895 Free PMC article.
References
-
- Nayak D.P. Virus Morphology, Replication, and Assembly. Viral Ecol. 2000:63–124. doi: 10.1016/B978-012362675-2/50004-5. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

