Retrospective analysis of pacritinib in patients with myelofibrosis and severe thrombocytopenia
- PMID: 34551507
- PMCID: PMC9244834
- DOI: 10.3324/haematol.2021.279415
Retrospective analysis of pacritinib in patients with myelofibrosis and severe thrombocytopenia
Abstract
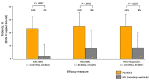
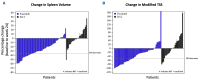
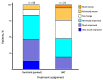
Thrombocytopenia is common in patients with myelofibrosis (MF) and is a well-established adverse prognostic factor. Both of the approved Janus kinase (JAK) inhibitors, ruxolitinib and fedratinib, can worsen thrombocytopenia and have not been evaluated in patients with severe thrombocytopenia (platelet counts <50×109/L). Pacritinib, a novel JAK2/interleukin-1 receptor-associated kinase 1 inhibitor, has been studied in two phase III trials (PERSIST-1 and PERSIST- 2), both of which enrolled patients with MF and severe thrombocytopenia. In order to better characterize treatment outcomes for this population with advanced disease, we present a retrospective analysis of efficacy and safety data in the 189 patients with severe thrombocytopenia treated in the PERSIST studies. The proportion of patients in the pacritinib group meeting efficacy endpoints was greater than in the BAT group for ≥35% spleen volume reduction (23% vs. 2%, P=0.0007), ≥50% modified Total Symptom Score reduction (25% vs. 8%, P=0.044), and self-reported symptom benefit ("much" or "very much" improved; 25% vs. 8%, P=0.016) at the primary analysis time point (week 24). The adverse event profile of pacritinib was manageable, and dose modification was rarely required. There was no excess in bleeding or death in pacritinib-treated patients. These results indicate that pacritinib is a promising treatment for patients with MF who lack safe and effective therapeutic options due to severe thrombocytopenia.
Trial registration: ClinicalTrials.gov NCT02055781 NCT04884191.
Figures

Similar articles
-
Pacritinib vs Best Available Therapy, Including Ruxolitinib, in Patients With Myelofibrosis: A Randomized Clinical Trial.JAMA Oncol. 2018 May 1;4(5):652-659. doi: 10.1001/jamaoncol.2017.5818. JAMA Oncol. 2018. PMID: 29522138 Free PMC article. Clinical Trial.
-
Pacritinib for the treatment of patients with myelofibrosis and thrombocytopenia.Expert Rev Hematol. 2022 Aug;15(8):671-684. doi: 10.1080/17474086.2022.2112565. Epub 2022 Sep 1. Expert Rev Hematol. 2022. PMID: 35983661 Review.
-
Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial.Lancet Haematol. 2017 May;4(5):e225-e236. doi: 10.1016/S2352-3026(17)30027-3. Epub 2017 Mar 20. Lancet Haematol. 2017. PMID: 28336242 Free PMC article. Clinical Trial.
-
Pacritinib to treat myelofibrosis patients with thrombocytopenia.Expert Rev Hematol. 2018 Sep;11(9):707-714. doi: 10.1080/17474086.2018.1500456. Epub 2018 Jul 19. Expert Rev Hematol. 2018. PMID: 30001163 Review.
-
Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies.J Hematol Oncol. 2016 Dec 8;9(1):137. doi: 10.1186/s13045-016-0367-x. J Hematol Oncol. 2016. PMID: 27931243 Free PMC article. Clinical Trial.
Cited by
-
Potential therapeutic targets of the JAK2/STAT3 signaling pathway in triple-negative breast cancer.Front Oncol. 2024 Apr 18;14:1381251. doi: 10.3389/fonc.2024.1381251. eCollection 2024. Front Oncol. 2024. PMID: 38699644 Free PMC article. Review.
-
Functional and Structural Characterization of Clinical-Stage Janus Kinase 2 Inhibitors Identifies Determinants for Drug Selectivity.J Med Chem. 2024 Jun 27;67(12):10012-10024. doi: 10.1021/acs.jmedchem.4c00197. Epub 2024 Jun 6. J Med Chem. 2024. PMID: 38843875 Free PMC article.
-
Pacritinib: First Approval.Drugs. 2022 May;82(7):831-838. doi: 10.1007/s40265-022-01718-y. Drugs. 2022. PMID: 35567653 Review.
-
JAK Inhibitors for Myelofibrosis: Strengths and Limitations.Curr Hematol Malig Rep. 2024 Dec;19(6):264-275. doi: 10.1007/s11899-024-00744-9. Epub 2024 Oct 14. Curr Hematol Malig Rep. 2024. PMID: 39400853 Free PMC article. Review.
-
The odyssey of pacritinib in myelofibrosis.Blood Adv. 2022 Aug 23;6(16):4905-4913. doi: 10.1182/bloodadvances.2022007524. Blood Adv. 2022. PMID: 35622972 Free PMC article.
References
-
- Hernandez-Boluda JC, Correa JG, Alvarez-Larran A, et al. . Clinical characteristics, prognosis and treatment of myelofibrosis patients with severe thrombocytopenia. Br J Haematol. 2018;181(3):397-400. - PubMed
-
- Jakafi (ruxolitinib) [package insert]. Wilmington, DE: Incyte; 2020.
-
- Inrebic (fedratinib) [package insert]. Summit, NJ: Celgene Corporation; 2019.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

