Tunisian Maturity-Onset Diabetes of the Young: A Short Review and a New Molecular and Clinical Investigation
- PMID: 34393998
- PMCID: PMC8358796
- DOI: 10.3389/fendo.2021.684018
Tunisian Maturity-Onset Diabetes of the Young: A Short Review and a New Molecular and Clinical Investigation
Abstract
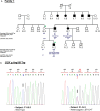
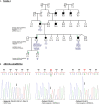
Introduction/aims: Maturity-Onset Diabetes of the Young (MODY) is a monogenic non-autoimmune diabetes with 14 different genetic forms. MODY-related mutations are rarely found in the Tunisian population. Here, we explored MODY related genes sequences among seventeen unrelated Tunisian probands qualifying the MODY clinical criteria.
Materials and methods: The GCK and HNF1A genes were systematically analyzed by direct sequencing in all probands. Then, clinical exome sequencing of 4,813 genes was performed on three unrelated patients. Among them, 130 genes have been reported to be involved in the regulation of glucose metabolism, β-cell development, differentiation and function. All identified variants were analyzed according to their frequencies in the GnomAD database and validated by direct sequencing.
Results: We identified the previously reported GCK mutation (rs1085307455) in one patient. The clinical features of the MODY2 proband were similar to previous reports. In this study, we revealed rare and novel alterations in GCK (rs780806456) and ABCC8 (rs201499958) genes with uncertain significance. We also found two likely benign alterations in HNF1A (rs1800574) and KLF11 (rs35927125) genes with minor allele frequencies similar to those depicted in public databases. No pathogenic variants have been identified through clinical exome analysis.
Conclusions: The most appropriate patients were selected, following a strict clinical screening approach, for genetic testing. However, the known MODY1-13 genes could not explain most of the Tunisian MODY cases, suggesting the involvement of unidentified genes in the majority of Tunisian affected families.
Keywords: GCK; HNF1A; MODY; Sanger sequencing; clinical exome sequencing; genetic testing.
Copyright © 2021 Moalla, Safi, Babiker Mansour, Hadj Kacem, Mahfood, Abid, Kammoun, Hachicha, Mnif-Feki, Hadj Kacem and Hadj Kacem.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Recognition of maturity-onset diabetes of the young in China.J Diabetes Investig. 2021 Apr;12(4):501-509. doi: 10.1111/jdi.13378. Epub 2020 Sep 9. J Diabetes Investig. 2021. PMID: 32741144 Free PMC article.
-
Maturity Onset Diabetes of the Young (MODY) in Tunisia: Low frequencies of GCK and HNF1A mutations.Gene. 2018 Apr 20;651:44-48. doi: 10.1016/j.gene.2018.01.081. Epub 2018 Feb 3. Gene. 2018. PMID: 29408271
-
Frequency and characterization of mutations in genes in a large cohort of patients referred to MODY registry.J Pediatr Endocrinol Metab. 2021 Apr 13;34(5):633-638. doi: 10.1515/jpem-2020-0501. Print 2021 May 26. J Pediatr Endocrinol Metab. 2021. PMID: 33852230 Free PMC article.
-
Novel insights into genetics and clinics of the HNF1A-MODY.Endocr Regul. 2019 Apr 1;53(2):110-134. doi: 10.2478/enr-2019-0013. Endocr Regul. 2019. PMID: 31517624 Review.
-
Maturity onset diabetes of the young: identification and diagnosis.Ann Clin Biochem. 2013 Sep;50(Pt 5):403-15. doi: 10.1177/0004563213483458. Epub 2013 Jul 22. Ann Clin Biochem. 2013. PMID: 23878349 Review.
Cited by
-
Treatment switch from multiple daily insulin injections to sulphonylureas in an African young adult diagnosed with HNF1A MODY: a case report.J Med Case Rep. 2024 Oct 17;18(1):506. doi: 10.1186/s13256-024-04850-3. J Med Case Rep. 2024. PMID: 39420387 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

