Fungal biosurfactants, from nature to biotechnological product: bioprospection, production and potential applications
- PMID: 34131819
- PMCID: PMC8205652
- DOI: 10.1007/s00449-021-02597-5
Fungal biosurfactants, from nature to biotechnological product: bioprospection, production and potential applications
Abstract
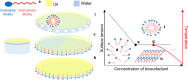
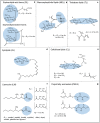
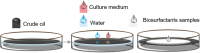
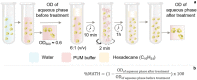
Biosurfactants are in demand by the global market as natural commodities that can be added to commercial products or use in environmental applications. These biomolecules reduce the surface/interfacial tension between fluid phases and exhibit superior stability to chemical surfactants under different physico-chemical conditions. Biotechnological production of biosurfactants is still emerging. Fungi are promising producers of these molecules with unique chemical structures, such as sophorolipids, mannosylerythritol lipids, cellobiose lipids, xylolipids, polyol lipids and hydrophobins. In this review, we aimed to contextualize concepts related to fungal biosurfactant production and its application in industry and the environment. Concepts related to the thermodynamic and physico-chemical properties of biosurfactants are presented, which allows detailed analysis of their structural and application. Promising niches for isolating biosurfactant-producing fungi are presented, as well as screening methodologies are discussed. Finally, strategies related to process parameters and variables, simultaneous production, process optimization through statistical and genetic tools, downstream processing and some aspects of commercial products formulations are presented.
Keywords: Bioprocess; Biosurfactants; Downstream; Filamentous fungi; Screening; Yeasts.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures





Similar articles
-
Current status in biotechnological production and applications of glycolipid biosurfactants.Appl Microbiol Biotechnol. 2016 Dec;100(24):10265-10293. doi: 10.1007/s00253-016-7980-z. Epub 2016 Nov 14. Appl Microbiol Biotechnol. 2016. PMID: 27844141 Review.
-
Biosurfactant production of Piper hispidum endophytic fungi.J Appl Microbiol. 2021 Feb;130(2):561-569. doi: 10.1111/jam.14398. Epub 2019 Aug 9. J Appl Microbiol. 2021. PMID: 31340085
-
Rhamnolipids--next generation surfactants?J Biotechnol. 2012 Dec 31;162(4):366-80. doi: 10.1016/j.jbiotec.2012.05.022. Epub 2012 Jun 20. J Biotechnol. 2012. PMID: 22728388 Review.
-
Overview on Glycosylated Lipids Produced by Bacteria and Fungi: Rhamno-, Sophoro-, Mannosylerythritol and Cellobiose Lipids.Adv Biochem Eng Biotechnol. 2022;181:73-122. doi: 10.1007/10_2021_200. Adv Biochem Eng Biotechnol. 2022. PMID: 35526186
-
Biosurfactants: a sustainable replacement for chemical surfactants?Biotechnol Lett. 2012 Sep;34(9):1597-605. doi: 10.1007/s10529-012-0956-x. Epub 2012 May 22. Biotechnol Lett. 2012. PMID: 22618240 Review.
Cited by
-
Visualizing liquid distribution across hyphal networks with cellular resolution.Biomicrofluidics. 2024 Oct 7;18(5):054109. doi: 10.1063/5.0231656. eCollection 2024 Sep. Biomicrofluidics. 2024. PMID: 39381835 Free PMC article.
-
Production and structural characterization of eco-friendly bioemulsifier SC04 from Saccharomyces cerevisiae strain MYN04 with potential applications.Microb Cell Fact. 2023 Sep 7;22(1):176. doi: 10.1186/s12934-023-02186-z. Microb Cell Fact. 2023. PMID: 37679768 Free PMC article.
-
Production and characterization of novel/chimeric sophorose-rhamnose biosurfactants by introducing heterologous rhamnosyltransferase genes into Starmerella bombicola.Biotechnol Biofuels Bioprod. 2024 Nov 5;17(1):133. doi: 10.1186/s13068-024-02581-7. Biotechnol Biofuels Bioprod. 2024. PMID: 39501413 Free PMC article.
-
Advancements in biosurfactant production using agro-industrial waste for industrial and environmental applications.Front Microbiol. 2024 Feb 5;15:1357302. doi: 10.3389/fmicb.2024.1357302. eCollection 2024. Front Microbiol. 2024. PMID: 38374917 Free PMC article. Review.
-
Biosurfactants: Forthcomings and Regulatory Affairs in Food-Based Industries.Molecules. 2023 Mar 21;28(6):2823. doi: 10.3390/molecules28062823. Molecules. 2023. PMID: 36985795 Free PMC article. Review.
References
-
- Çelik PA, Manga EB, Çabuk A, Banat IM. Biosurfactants’ potential role in combating COVID-19 and similar future microbial threats. Appl Sci. 2020;11:334. doi: 10.3390/app11010334. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

