The Stress-Responsive Alternative Sigma Factor SigB of Bacillus subtilis and Its Relatives: An Old Friend With New Functions
- PMID: 33042030
- PMCID: PMC7522486
- DOI: 10.3389/fmicb.2020.01761
The Stress-Responsive Alternative Sigma Factor SigB of Bacillus subtilis and Its Relatives: An Old Friend With New Functions
Abstract
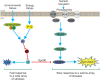
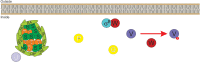
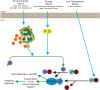
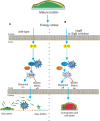
Alternative sigma factors have led the core RNA polymerase (RNAP) to recognize different sets of promoters to those recognized by the housekeeping sigma A-directed RNAP. This change in RNAP promoter selectivity allows a rapid and flexible reformulation of the genetic program to face environmental and metabolic stimuli that could compromise bacterial fitness. The model bacterium Bacillus subtilis constitutes a matchless living system in the study of the role of alternative sigma factors in gene regulation and physiology. SigB from B. subtilis was the first alternative sigma factor described in bacteria. Studies of SigB during the last 40 years have shown that it controls a genetic universe of more than 150 genes playing crucial roles in stress response, adaption, and survival. Activation of SigB relies on three separate pathways that specifically respond to energy, environmental, and low temperature stresses. SigB homologs, present in other Gram-positive bacteria, also play important roles in virulence against mammals. Interestingly, during recent years, other unexpected B. subtilis responses were found to be controlled by SigB. In particular, SigB controls the efficiencies of spore and biofilm formation, two important features that play critical roles in adaptation and survival in planktonic and sessile B. subtilis communities. In B. subtilis, SigB induces the expression of the Spo0E aspartyl-phosphatase, which is responsible for the blockage of sporulation initiation. The upregulated activity of Spo0E connects the two predominant adaptive pathways (i.e., sporulation and stress response) present in B. subtilis. In addition, the RsbP serine-phosphatase, belonging to the energy stress arm of the SigB regulatory cascade, controls the expression of the key transcription factor SinR to decide whether cells residing in the biofilm remain in and maintain biofilm growth or scape to colonize new niches through biofilm dispersal. SigB also intervenes in the recognition of and response to surrounding microorganisms, a new SigB role that could have an agronomic impact. SigB is induced when B. subtilis is confronted with phytopathogenic fungi (e.g., Fusarium verticillioides) and halts fungal growth to the benefit of plant growth. In this article, we update and review literature on the different regulatory networks that control the activation of SigB and the new roles that have been described the recent years.
Keywords: Bacillus subtilis; SigB; alternative sigma factors; biocontrol; biofilm fitness; general stress response; sporulation.
Copyright © 2020 Rodriguez Ayala, Bartolini and Grau.
Figures

Similar articles
-
Regulation of Biofilm Aging and Dispersal in Bacillus subtilis by the Alternative Sigma Factor SigB.J Bacteriol. 2018 Dec 20;201(2):e00473-18. doi: 10.1128/JB.00473-18. Print 2019 Jan 15. J Bacteriol. 2018. PMID: 30396900 Free PMC article.
-
Stress-Responsive Alternative Sigma Factor SigB Plays a Positive Role in the Antifungal Proficiency of Bacillus subtilis.Appl Environ Microbiol. 2019 Apr 18;85(9):e00178-19. doi: 10.1128/AEM.00178-19. Print 2019 May 1. Appl Environ Microbiol. 2019. PMID: 30824454 Free PMC article.
-
RsbV-independent induction of the SigB-dependent general stress regulon of Bacillus subtilis during growth at high temperature.J Bacteriol. 2004 Sep;186(18):6150-8. doi: 10.1128/JB.186.18.6150-6158.2004. J Bacteriol. 2004. PMID: 15342585 Free PMC article.
-
SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria.Annu Rev Microbiol. 2007;61:215-36. doi: 10.1146/annurev.micro.61.080706.093445. Annu Rev Microbiol. 2007. PMID: 18035607 Review.
-
General stress response of Bacillus subtilis and other bacteria.Adv Microb Physiol. 2001;44:35-91. doi: 10.1016/s0065-2911(01)44011-2. Adv Microb Physiol. 2001. PMID: 11407115 Review.
Cited by
-
The Transcriptional Regulatory Network of Corynebacterium pseudotuberculosis.Microorganisms. 2021 Feb 17;9(2):415. doi: 10.3390/microorganisms9020415. Microorganisms. 2021. PMID: 33671149 Free PMC article. Review.
-
Transcriptomic analysis of the antimicrobial activity of prodigiosin against Cutibacterium acnes.Sci Rep. 2023 Oct 13;13(1):17412. doi: 10.1038/s41598-023-44612-7. Sci Rep. 2023. PMID: 37833344 Free PMC article.
-
The σB alternative sigma factor circuit modulates noise to generate different types of pulsing dynamics.PLoS Comput Biol. 2023 Aug 4;19(8):e1011265. doi: 10.1371/journal.pcbi.1011265. eCollection 2023 Aug. PLoS Comput Biol. 2023. PMID: 37540712 Free PMC article.
-
Design-build-test of recombinant Bacillus subtilis chassis cell by lifespan engineering for robust bioprocesses.Synth Syst Biotechnol. 2024 Apr 11;9(3):470-480. doi: 10.1016/j.synbio.2024.04.004. eCollection 2024 Sep. Synth Syst Biotechnol. 2024. PMID: 38634000 Free PMC article.
-
Proteomic analysis to unravel the biochemical mechanisms triggered by Bacillus toyonensis SFC 500-1E under chromium(VI) and phenol stress.Biometals. 2023 Oct;36(5):1081-1108. doi: 10.1007/s10534-023-00506-9. Epub 2023 May 20. Biometals. 2023. PMID: 37209221 Review.
References
-
- Akbar S., Gaidenko T. A., Kang C. M., O’Reilly M., Devine K. M., Price C. W. (2001). New family of regulators in the environmental signaling pathway which activates the general stress transcription factor sigma (B) of Bacillus subtilis. J Bacteriol. 183 1329–1338. 10.1128/jb.183.4.1329-1338.2001 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

