Function of miR825 and miR825* as Negative Regulators in Bacillus cereus AR156-elicited Systemic Resistance to Botrytis cinerea in Arabidopsis thaliana
- PMID: 31614458
- PMCID: PMC6829492
- DOI: 10.3390/ijms20205032
Function of miR825 and miR825* as Negative Regulators in Bacillus cereus AR156-elicited Systemic Resistance to Botrytis cinerea in Arabidopsis thaliana
Abstract
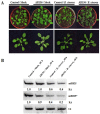
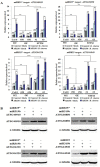
Small RNAs function to regulate plant defense responses to pathogens. We previously showed that miR825 and miR825* downregulate Bacillus cereus AR156 (AR156)-triggered systemic resistance to Pseudomonassyringae pv. tomato DC3000 in Arabidopsis thaliana (Arabidopsis). Here, Northern blotting revealed that miR825 and miR825* were more strongly downregulated in wild type Arabidopsis Col-0 (Col-0) plants pretreated with AR156 than in nontreated plants upon Botrytis cinerea (B. cinerea) B1301 infection. Furthermore, compared with Col-0, transgenic plants with attenuated miR825 and miR825* expression were more resistant to B. cinerea B1301, yet miR825- and miR825*-overexpressing (OE) plants were more susceptible to the pathogen. With AR156 pretreatment, the transcription of four defense-related genes (PR1, PR2, PR5, and PDF1.2) and cellular defense responses (hydrogen peroxide production and callose deposition) were faster and stronger in miR825 and miR825* knockdown lines but weaker in their OE plants than in Col-0 plants upon pathogen attack. Also, AR156 pretreatment caused stronger phosphorylation of MPK3 and MPK6 and expression of FRK1 and WRKY53 genes upon B. cinerea B1301 inoculation in miR825 and miR825* knockdown plants than in Col-0 plants. Additionally, the assay of agrobacterium-mediated transient co-expression in Nicotiana benthamiana confirmed that AT5G40910, AT5G38850, AT3G04220, and AT5G44940 are target genes of miR825 or miR825*. Compared with Col-0, the target mutant lines showed higher susceptibility to B. cinerea B1301, while still expressing AR156-triggered induced systemic resistance (ISR). The two-way analysis of variance (ANOVA) revealed a significant (P < 0.01) interactive effect of treatment and genotype on the defense responses. Hence, miR825 and miR825*act as negative regulators of AR156-mediated systemic resistance to B. cinerea B1301 in Arabidopsis.
Keywords: Bacillus cereus AR156; Botrytis cinerea B1301; induced systemic resistance; miR825 and miR825*; plant innate immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Bacillus cereus AR156 primes induced systemic resistance by suppressing miR825/825* and activating defense-related genes in Arabidopsis.J Integr Plant Biol. 2016 Apr;58(4):426-39. doi: 10.1111/jipb.12446. Epub 2016 Feb 5. J Integr Plant Biol. 2016. PMID: 26526683 Free PMC article.
-
The plant growth-promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate- and jasmonate/ethylene-dependent signaling pathways.Mol Plant Microbe Interact. 2011 May;24(5):533-42. doi: 10.1094/MPMI-09-10-0213. Mol Plant Microbe Interact. 2011. PMID: 21198361
-
Bacillus cereus AR156 triggers induced systemic resistance against Pseudomonas syringae pv. tomato DC3000 by suppressing miR472 and activating CNLs-mediated basal immunity in Arabidopsis.Mol Plant Pathol. 2020 Jun;21(6):854-870. doi: 10.1111/mpp.12935. Epub 2020 Mar 30. Mol Plant Pathol. 2020. PMID: 32227587 Free PMC article.
-
Botrytis small RNA Bc-siR37 suppresses plant defense genes by cross-kingdom RNAi.RNA Biol. 2017 Apr 3;14(4):421-428. doi: 10.1080/15476286.2017.1291112. Epub 2017 Mar 7. RNA Biol. 2017. PMID: 28267415 Free PMC article. Review.
-
Plant Growth Promotion Using Bacillus cereus.Int J Mol Sci. 2023 Jun 5;24(11):9759. doi: 10.3390/ijms24119759. Int J Mol Sci. 2023. PMID: 37298706 Free PMC article. Review.
Cited by
-
Identification of miRNAs Involved in Maize-Induced Systemic Resistance Primed by Trichoderma harzianum T28 against Cochliobolus heterostrophus.J Fungi (Basel). 2023 Feb 20;9(2):278. doi: 10.3390/jof9020278. J Fungi (Basel). 2023. PMID: 36836392 Free PMC article.
-
Bacillus subtilis 26D Triggers Induced Systemic Resistance against Rhopalosiphum padi L. by Regulating the Expression of Genes AGO, DCL and microRNA in Bread Spring Wheat.Microorganisms. 2023 Dec 14;11(12):2983. doi: 10.3390/microorganisms11122983. Microorganisms. 2023. PMID: 38138127 Free PMC article.
-
Biocontrol of fungal phytopathogens by Bacillus pumilus.Front Microbiol. 2023 Jul 25;14:1194606. doi: 10.3389/fmicb.2023.1194606. eCollection 2023. Front Microbiol. 2023. PMID: 37560520 Free PMC article. Review.
-
Plant-Associated Bacillus thuringiensis and Bacillus cereus: Inside Agents for Biocontrol and Genetic Recombination in Phytomicrobiome.Plants (Basel). 2023 Nov 30;12(23):4037. doi: 10.3390/plants12234037. Plants (Basel). 2023. PMID: 38068672 Free PMC article. Review.
-
RcTGA1 and glucosinolate biosynthesis pathway involvement in the defence of rose against the necrotrophic fungus Botrytis cinerea.BMC Plant Biol. 2021 May 17;21(1):223. doi: 10.1186/s12870-021-02973-z. BMC Plant Biol. 2021. PMID: 34001006 Free PMC article.
References
-
- Ichimura K., Shinozaki K., Tena G., Sheen J., Henry Y., Champion A., Kreis M., Zhang S.Q., Hirt H., Wilson C., et al. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002;7:301–308. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

