Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy
- PMID: 31356140
- PMCID: PMC6784850
- DOI: 10.1200/JCO.19.01140
Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy
Abstract
Purpose: Locally advanced or metastatic urothelial carcinoma is an incurable disease with limited treatment options, especially for patients who were previously treated with platinum and anti-programmed death 1 or anti-programmed death ligand 1 (PD-1/L1) therapy. Enfortumab vedotin is an antibody-drug conjugate that targets Nectin-4, which is highly expressed in urothelial carcinoma.
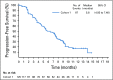
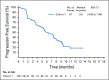
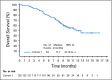
Methods: EV-201 is a global, phase II, single-arm study of enfortumab vedotin 1.25 mg/kg (intravenously on days 1, 8, and 15 of every 28-day cycle) in patients with locally advanced or metastatic urothelial carcinoma who were previously treated with platinum chemotherapy and anti-PD-1/L1 therapy. The primary end point was objective response rate per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 by blinded independent central review. Key secondary end points were duration of response, progression-free survival, overall survival, safety, and tolerability.
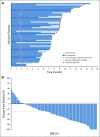
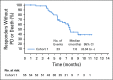
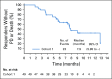
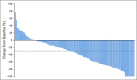
Results: Enfortumab vedotin was administered to 125 patients with metastatic urothelial carcinoma. Median follow-up was 10.2 months (range, 0.5 to 16.5 months). Confirmed objective response rate was 44% (95% CI, 35.1% to 53.2%), including 12% complete responses. Similar responses were observed in prespecified subgroups, such as those patients with liver metastases and those with no response to prior anti-PD-1/L1 therapy. Median duration of response was 7.6 months (range, 0.95 to 11.30+ months). The most common treatment-related adverse events were fatigue (50%), any peripheral neuropathy (50%), alopecia (49%), any rash (48%), decreased appetite (44%), and dysgeusia (40%). No single treatment-related adverse events grade 3 or greater occurred in 10% or more of patients.
Conclusion: Enfortumab vedotin demonstrated a clinically meaningful response rate with a manageable and tolerable safety profile in patients with locally advanced or metastatic urothelial carcinoma who were previously treated with platinum and anti-PD-1/L1 therapies.
Trial registration: ClinicalTrials.gov NCT03219333.
Figures

Comment in
-
Immunotherapy for Urothelial Cancer: Where Are the Randomized Trials?J Clin Oncol. 2019 Oct 10;37(29):2587-2591. doi: 10.1200/JCO.18.02257. Epub 2019 Jul 1. J Clin Oncol. 2019. PMID: 31260641 No abstract available.
-
New targeted agents for urothelial carcinoma.Nat Rev Clin Oncol. 2019 Oct;16(10):591. doi: 10.1038/s41571-019-0263-8. Nat Rev Clin Oncol. 2019. PMID: 31406342 No abstract available.
-
Enfortumab Vedotin Checks Urothelial Cancer.Cancer Discov. 2019 Oct;9(10):OF1. doi: 10.1158/2159-8290.CD-NB2019-104. Epub 2019 Sep 13. Cancer Discov. 2019. PMID: 31519705
-
Value of Biomarker Expression for Randomized Clinical Trial Design: One (More) Missed Opportunity.J Clin Oncol. 2020 Feb 20;38(6):649-651. doi: 10.1200/JCO.19.02393. Epub 2019 Dec 27. J Clin Oncol. 2020. PMID: 31880965 No abstract available.
-
Reply to F.E. Vera-Badillo et al.J Clin Oncol. 2020 Feb 20;38(6):651-652. doi: 10.1200/JCO.19.02830. Epub 2019 Dec 27. J Clin Oncol. 2020. PMID: 31880968 No abstract available.
-
Enfortumab vedotin shows promise in solid tumours.Lancet Oncol. 2020 Mar;21(3):e133. doi: 10.1016/S1470-2045(20)30089-9. Epub 2020 Feb 14. Lancet Oncol. 2020. PMID: 32066546 No abstract available.
Similar articles
-
Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV‑201): a multicentre, single-arm, phase 2 trial.Lancet Oncol. 2021 Jun;22(6):872-882. doi: 10.1016/S1470-2045(21)00094-2. Epub 2021 May 12. Lancet Oncol. 2021. PMID: 33991512 Clinical Trial.
-
Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma.N Engl J Med. 2021 Mar 25;384(12):1125-1135. doi: 10.1056/NEJMoa2035807. Epub 2021 Feb 12. N Engl J Med. 2021. PMID: 33577729 Free PMC article. Clinical Trial.
-
Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial.Lancet Oncol. 2018 Jan;19(1):51-64. doi: 10.1016/S1470-2045(17)30900-2. Epub 2017 Dec 5. Lancet Oncol. 2018. PMID: 29217288 Free PMC article. Clinical Trial.
-
Enfortumab vedotin to treat urothelial carcinoma.Drugs Today (Barc). 2020 May;56(5):329-335. doi: 10.1358/dot.2020.56.5.3127027. Drugs Today (Barc). 2020. PMID: 32406880 Review.
-
Clinical Pharmacology of the Antibody-Drug Conjugate Enfortumab Vedotin in Advanced Urothelial Carcinoma and Other Malignant Solid Tumors.Clin Pharmacokinet. 2024 Apr;63(4):423-438. doi: 10.1007/s40262-024-01369-0. Epub 2024 Apr 12. Clin Pharmacokinet. 2024. PMID: 38609704 Free PMC article. Review.
Cited by
-
New and Emerging Therapies in the Management of Bladder Cancer.F1000Res. 2020 Sep 16;9:F1000 Faculty Rev-1146. doi: 10.12688/f1000research.26841.1. eCollection 2020. F1000Res. 2020. PMID: 32983413 Free PMC article. Review.
-
Early Bone Metastases are Associated with Worse Outcomes in Metastatic Urothelial Carcinoma.Bladder Cancer. 2021 Mar 19;7(1):33-42. doi: 10.3233/BLC-200377. eCollection 2021. Bladder Cancer. 2021. PMID: 38993215 Free PMC article.
-
Immunotherapy in non-muscle-invasive bladder cancer: current status and future directions.World J Urol. 2021 May;39(5):1319-1329. doi: 10.1007/s00345-020-03474-8. Epub 2020 Oct 15. World J Urol. 2021. PMID: 33057888 Review.
-
Effect of Enfortumab Vedotin Dose Adjustment on Efficacy in Metastatic Urothelial Carcinoma: A Retrospective Single-Center Experience .Cancer Manag Res. 2023 Nov 6;15:1245-1250. doi: 10.2147/CMAR.S424070. eCollection 2023. Cancer Manag Res. 2023. PMID: 37953888 Free PMC article. No abstract available.
-
Real-World Insights into Efficacy and Safety of Enfortumab Vedotin in Japanese Patients with Metastatic Urothelial Carcinoma: Findings, Considerations, and Future Directions.Curr Oncol. 2024 Jan 29;31(2):759-768. doi: 10.3390/curroncol31020056. Curr Oncol. 2024. PMID: 38392050 Free PMC article.
References
-
- National Cancer Institute . SEER Cancer Stat Facts: Bladder Cancer. Bethesda, MD: National Cancer Institute; 2019.
-
- von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: Results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. - PubMed
-
- De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–199. - PMC - PubMed
-
- Bellmunt J, von der Maase H, Mead GM, et al. Randomized phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC Intergroup Study 30987. J Clin Oncol. 2012;30:1107–1113. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

