Updated clinical guidelines experience major reporting limitations
- PMID: 29025429
- PMCID: PMC5639761
- DOI: 10.1186/s13012-017-0651-3
Updated clinical guidelines experience major reporting limitations
Erratum in
-
Correction to: Updated clinical guidelines experience major reporting limitations.Implement Sci. 2018 Apr 30;13(1):64. doi: 10.1186/s13012-018-0759-0. Implement Sci. 2018. PMID: 29712556 Free PMC article.
Abstract
Background: The Checklist for the Reporting of Updated Guidelines (CheckUp) was recently developed. However, so far, no systematic assessment of the reporting of updated clinical guidelines (CGs) exists. We aimed to examine (1) the completeness of reporting the updating process in CGs and (2) the inter-observer reliability of CheckUp.
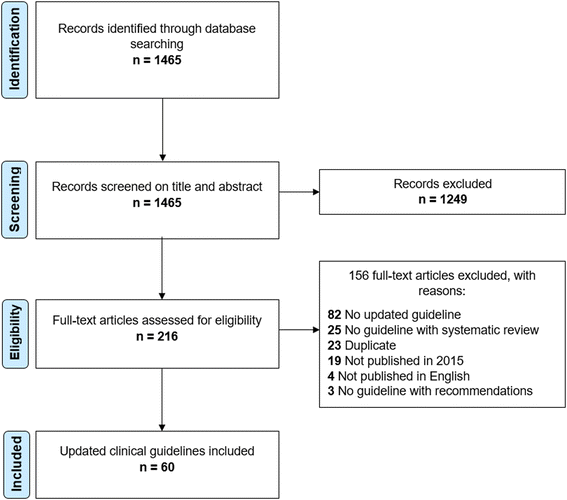
Methods: We conducted a systematic assessment of the reporting of the updating process in a sample of updated CGs using CheckUp. We performed a systematic search to identify updated CGs published in 2015, developed by a professional society, reporting a systematic review of the evidence, and containing at least one recommendation. Three reviewers independently assessed the CGs with CheckUp (16 items). We calculated the median score per item, per domain, and overall, converting scores to a 10-point scale. Multiple linear regression analyses were used to identify differences according to country, type of organisation, scope, and health topic of updated CGs. We calculated the intraclass coefficient (ICC) and 95% confidence interval (95% CI) for domains and overall score.
Results: We included in total 60 updated CGs. The median domain score on a 10-point scale for presentation was 5.8 (range 1.7 to 10), for editorial independence 8.3 (range 3.3 to 10), and for methodology 5.7 (range 0 to 10). The median overall score on a 10-point scale was 6.3 (range 3.1 to 10). Presentation and justification items at recommendation level (respectively reported by 27 and 38% of the CGs) and the methods used for the external review and implementing changes in practice were particularly poorly reported (both reported by 38% of the CGs). CGs developed by a European or international institution obtained a statistically significant higher overall score compared to North American or Asian institutions (p = 0.014). Finally, the agreement among the reviewers on the overall score was excellent (ICC 0.88, 95% CI 0.75 to 0.95).
Conclusions: The reporting of updated CGs varies considerably with significant room for improvement. We recommend using CheckUp to assess the updating process in updated CGs and as a blueprint to inform methods and reporting strategies in updating.
Keywords: Checklist/standards; Guideline [publication type]; Publishing/standards.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp).PLoS Med. 2017 Jan 10;14(1):e1002207. doi: 10.1371/journal.pmed.1002207. eCollection 2017 Jan. PLoS Med. 2017. PMID: 28072838 Free PMC article.
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Updated recommendations: an assessment of NICE clinical guidelines.Implement Sci. 2014 Jun 11;9:72. doi: 10.1186/1748-5908-9-72. Implement Sci. 2014. PMID: 24919856 Free PMC article.
-
Updating Systematic Reviews.Rockville (MD): Agency for Healthcare Research and Quality (US); 2007 Sep. Report No.: 07-0087. Rockville (MD): Agency for Healthcare Research and Quality (US); 2007 Sep. Report No.: 07-0087. PMID: 20734512 Free Books & Documents. Review.
Cited by
-
Correction to: Updated clinical guidelines experience major reporting limitations.Implement Sci. 2018 Apr 30;13(1):64. doi: 10.1186/s13012-018-0759-0. Implement Sci. 2018. PMID: 29712556 Free PMC article.
-
Optimizing a literature surveillance strategy to retrieve sound overall prognosis and risk assessment model papers.J Am Med Inform Assoc. 2021 Mar 18;28(4):766-771. doi: 10.1093/jamia/ocaa232. J Am Med Inform Assoc. 2021. PMID: 33484123 Free PMC article.
-
Using RIGHT (Reporting Items for Practice Guidelines in Healthcare) to evaluate the reporting quality of WHO guidelines.Health Res Policy Syst. 2020 Jul 8;18(1):75. doi: 10.1186/s12961-020-00578-w. Health Res Policy Syst. 2020. PMID: 32641144 Free PMC article.
-
The reporting checklist for public versions of guidelines: RIGHT-PVG.Implement Sci. 2021 Jan 11;16(1):10. doi: 10.1186/s13012-020-01066-z. Implement Sci. 2021. PMID: 33430911 Free PMC article. Review.
-
World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines update - IV - A quality appraisal with the AGREE II instrument.World Allergy Organ J. 2022 Mar 2;15(2):100613. doi: 10.1016/j.waojou.2021.100613. eCollection 2022 Feb. World Allergy Organ J. 2022. PMID: 36091188 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources