Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial
- PMID: 28336242
- PMCID: PMC8209752
- DOI: 10.1016/S2352-3026(17)30027-3
Pacritinib versus best available therapy for the treatment of myelofibrosis irrespective of baseline cytopenias (PERSIST-1): an international, randomised, phase 3 trial
Abstract
Background: Available therapies for myelofibrosis can exacerbate cytopenias and are not indicated for patients with severe thrombocytopenia. Pacritinib, which inhibits both JAK2 and FLT3, induced spleen responses with limited myelosuppression in phase 1/2 trials. We aimed to assess the efficacy and safety of pacritinib versus best available therapy in patients with myelofibrosis irrespective of baseline cytopenias.
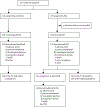
Methods: This international, multicentre, randomised, phase 3 trial (PERSIST-1) was done at 67 sites in 12 countries. Patients with higher-risk myelofibrosis (with no exclusions for baseline anaemia or thrombocytopenia) were randomly assigned (2:1) to receive oral pacritinib 400 mg once daily or best available therapy (BAT) excluding JAK2 inhibitors until disease progression or unacceptable toxicity. Randomisation was stratified by risk category, platelet count, and region. Treatment assignments were known to investigators, site personnel, patients, clinical monitors, and pharmacovigilance personnel. The primary endpoint was spleen volume reduction (SVR) of 35% or more from baseline to week 24 in the intention-to-treat population as assessed by blinded, centrally reviewed MRI or CT. We did safety analyses in all randomised patients who received either treatment. Here we present the final data. This trial is registered with ClinicalTrials.gov, number NCT01773187.
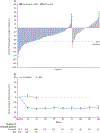
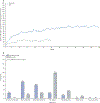
Findings: Between Jan 8, 2013, and Aug 1, 2014, 327 patients were randomly assigned to pacritinib (n=220) or BAT (n=107). Median follow-up was 23·2 months (IQR 14·8-28·7). At week 24, the primary endpoint of SVR of 35% or more was achieved by 42 (19%) patients in the pacritinib group versus five (5%) patients in the BAT group (p=0·0003). 90 patients in the BAT group crossed over to receive pacritinib at a median of 6·3 months (IQR 5·8-6·7). The most common grade 3-4 adverse events through week 24 were anaemia (n=37 [17%]), thrombocytopenia (n=26 [12%]), and diarrhoea (n=11 [5%]) in the pacritinib group, and anaemia (n=16 [15%]), thrombocytopenia (n=12 [11%]), dyspnoea (n=3 [3%]), and hypotension (n=3 [3%]) in the BAT group. The most common serious adverse events that occurred through week 24 were anaemia (10 [5%]), cardiac failure (5 [2%]), pyrexia (4 [2%]), and pneumonia (4 [2%]) with pacritinib, and anaemia (5 [5%]), sepsis (2 [2%]), and dyspnoea (2 [2%]) with BAT. Deaths due to adverse events were observed in 27 (12%) patients in the pacritinib group and 14 (13%) patients in the BAT group throughout the duration of the study.
Interpretation: Pacritinib therapy was well tolerated and induced significant and sustained SVR and symptom reduction, even in patients with severe baseline cytopenias. Pacritinib could be a treatment option for patients with myelofibrosis, including those with baseline cytopenias for whom options are particularly limited.
Funding: CTI BioPharma Corp.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The remaining authors declare no competing interests.
Figures

Comment in
-
Authors' reply to comment What should be the endpoints of a symptomatic drug and its control?Lancet Haematol. 2017 Nov;4(11):e508-e509. doi: 10.1016/S2352-3026(17)30195-3. Lancet Haematol. 2017. PMID: 29100557 No abstract available.
Similar articles
-
Pacritinib vs Best Available Therapy, Including Ruxolitinib, in Patients With Myelofibrosis: A Randomized Clinical Trial.JAMA Oncol. 2018 May 1;4(5):652-659. doi: 10.1001/jamaoncol.2017.5818. JAMA Oncol. 2018. PMID: 29522138 Free PMC article. Clinical Trial.
-
Phase 1/2 study of pacritinib, a next generation JAK2/FLT3 inhibitor, in myelofibrosis or other myeloid malignancies.J Hematol Oncol. 2016 Dec 8;9(1):137. doi: 10.1186/s13045-016-0367-x. J Hematol Oncol. 2016. PMID: 27931243 Free PMC article. Clinical Trial.
-
Pacritinib to treat myelofibrosis patients with thrombocytopenia.Expert Rev Hematol. 2018 Sep;11(9):707-714. doi: 10.1080/17474086.2018.1500456. Epub 2018 Jul 19. Expert Rev Hematol. 2018. PMID: 30001163 Review.
-
Momelotinib versus best available therapy in patients with myelofibrosis previously treated with ruxolitinib (SIMPLIFY 2): a randomised, open-label, phase 3 trial.Lancet Haematol. 2018 Feb;5(2):e73-e81. doi: 10.1016/S2352-3026(17)30237-5. Epub 2017 Dec 20. Lancet Haematol. 2018. PMID: 29275119 Clinical Trial.
-
The development, safety and efficacy of pacritinib for the treatment of myelofibrosis.Expert Rev Anticancer Ther. 2016 Nov;16(11):1101-1108. doi: 10.1080/14737140.2016.1233061. Epub 2016 Sep 21. Expert Rev Anticancer Ther. 2016. PMID: 27598824 Review.
Cited by
-
Comparative efficacy and hematologic safety of different dosages of JAK inhibitors in the treatment of myelofibrosis: a network meta-analysis.Front Oncol. 2024 Aug 30;14:1403967. doi: 10.3389/fonc.2024.1403967. eCollection 2024. Front Oncol. 2024. PMID: 39281381 Free PMC article.
-
FLT3-TKD in the prognosis of patients with acute myeloid leukemia: A meta-analysis.Front Oncol. 2023 Feb 17;13:1086846. doi: 10.3389/fonc.2023.1086846. eCollection 2023. Front Oncol. 2023. PMID: 36874106 Free PMC article.
-
An Insight into the Medicinal Chemistry Perspective of Macrocyclic Derivatives with Antitumor Activity: A Systematic Review.Molecules. 2022 Apr 29;27(9):2837. doi: 10.3390/molecules27092837. Molecules. 2022. PMID: 35566196 Free PMC article. Review.
-
Patterns of Ruxolitinib Therapy Failure and Its Management in Myelofibrosis: Perspectives of the Canadian Myeloproliferative Neoplasm Group.JCO Oncol Pract. 2020 Jul;16(7):351-359. doi: 10.1200/JOP.19.00506. Epub 2020 Mar 5. JCO Oncol Pract. 2020. PMID: 32134707 Free PMC article. Review.
-
SOHO State of the Art Updates and Next Questions: Identifying and Treating "Progression" in Myelofibrosis.Clin Lymphoma Myeloma Leuk. 2021 Oct;21(10):641-649. doi: 10.1016/j.clml.2021.06.008. Epub 2021 Jun 23. Clin Lymphoma Myeloma Leuk. 2021. PMID: 34272171 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

