FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review)
- PMID: 27245147
- PMCID: PMC4899036
- DOI: 10.3892/ijmm.2016.2620
FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review)
Abstract
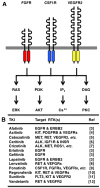
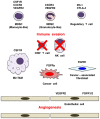
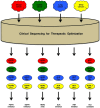
Fibroblast growth factor (FGF)2, FGF4, FGF7 and FGF20 are representative paracrine FGFs binding to heparan-sulfate proteoglycan and fibroblast growth factor receptors (FGFRs), whereas FGF19, FGF21 and FGF23 are endocrine FGFs binding to Klotho and FGFRs. FGFR1 is relatively frequently amplified and overexpressed in breast and lung cancer, and FGFR2 in gastric cancer. BCR-FGFR1, CNTRL-FGFR1, CUX1-FGFR1, FGFR1OP-FGFR1, MYO18A-FGFR1 and ZMYM2-FGFR1 fusions in myeloproliferative neoplasms are non-receptor-type FGFR kinases, whereas FGFR1-TACC1, FGFR2-AFF3, FGFR2-BICC1, FGFR2-PPHLN1, FGFR3-BAIAP2L1 and FGFR3-TACC3 fusions in solid tumors are transmembrane-type FGFRs with C-terminal alterations. AZD4547, BGJ398 (infigratinib), Debio-1347 and dovitinib are FGFR1/2/3 inhibitors; BLU9931 is a selective FGFR4 inhibitor; FIIN-2, JNJ-42756493, LY2874455 and ponatinib are pan-FGFR inhibitors. AZD4547, dovitinib and ponatinib are multi-kinase inhibitors targeting FGFRs, colony stimulating factor 1 receptor (CSF1R), vascular endothelial growth factor (VEGF)R2, and others. The tumor microenvironment consists of cancer cells and stromal/immune cells, such as cancer-associated fibroblasts (CAFs), endothelial cells, M2-type tumor-associating macrophages (M2-TAMs), myeloid-derived suppressor cells (MDSCs) and regulatory T cells. FGFR inhibitors elicit antitumor effects directly on cancer cells, as well as indirectly through the blockade of paracrine signaling. The dual inhibition of FGF and CSF1 or VEGF signaling is expected to enhance the antitumor effects through the targeting of immune evasion and angiogenesis in the tumor microenvironment. Combination therapy using tyrosine kinase inhibitors (FGFR or CSF1R inhibitors) and immune checkpoint blockers (anti-PD-1 or anti-CTLA-4 monoclonal antibodies) may be a promising choice for cancer patients. The inhibition of FGF19-FGFR4 signaling is associated with a risk of liver toxicity, whereas the activation of FGF23-FGFR4 signaling is associated with a risk of heart toxicity. Endocrine FGF signaling affects the pathophysiology of cancer patients who are prescribed FGFR inhibitors. Whole-genome sequencing is necessary for the detection of promoter/enhancer alterations of FGFR genes and rare alterations of other genes causing FGFR overexpression. To sustain the health care system in an aging society, a benefit-cost analysis should be performed with a focus on disease-free survival and the total medical cost before implementing genome-based precision medicine for cancer patients.
Figures

Similar articles
-
FGF receptors: cancer biology and therapeutics.Med Res Rev. 2014 Mar;34(2):280-300. doi: 10.1002/med.21288. Epub 2013 May 21. Med Res Rev. 2014. PMID: 23696246 Review.
-
The role of fibroblast growth factor receptor (FGFR) protein-tyrosine kinase inhibitors in the treatment of cancers including those of the urinary bladder.Pharmacol Res. 2020 Jan;151:104567. doi: 10.1016/j.phrs.2019.104567. Epub 2019 Nov 23. Pharmacol Res. 2020. PMID: 31770593 Review.
-
Evaluation of FGFR targeting in breast cancer through interrogation of patient-derived models.Breast Cancer Res. 2021 Aug 3;23(1):82. doi: 10.1186/s13058-021-01461-4. Breast Cancer Res. 2021. PMID: 34344433 Free PMC article.
-
Preclinical evaluation of 3D185, a novel potent inhibitor of FGFR1/2/3 and CSF-1R, in FGFR-dependent and macrophage-dominant cancer models.J Exp Clin Cancer Res. 2019 Aug 22;38(1):372. doi: 10.1186/s13046-019-1357-y. J Exp Clin Cancer Res. 2019. PMID: 31438996 Free PMC article.
-
Molecular subclasses of hepatocellular carcinoma predict sensitivity to fibroblast growth factor receptor inhibition.Int J Cancer. 2016 Mar 15;138(6):1494-505. doi: 10.1002/ijc.29893. Epub 2015 Nov 9. Int J Cancer. 2016. PMID: 26481559 Free PMC article.
Cited by
-
Inhibition of FGF-FGFR and VEGF-VEGFR signalling in cancer treatment.Cell Prolif. 2021 Apr;54(4):e13009. doi: 10.1111/cpr.13009. Epub 2021 Mar 2. Cell Prolif. 2021. PMID: 33655556 Free PMC article. Review.
-
First-In-Human Phase I Study of Tinengotinib (TT-00420), a Multiple Kinase Inhibitor, as a Single Agent in Patients With Advanced Solid Tumors.Oncologist. 2024 Apr 4;29(4):e514-e525. doi: 10.1093/oncolo/oyad338. Oncologist. 2024. PMID: 38297981 Free PMC article. Clinical Trial.
-
Structure, activation and dysregulation of fibroblast growth factor receptor kinases: perspectives for clinical targeting.Biochem Soc Trans. 2018 Dec 17;46(6):1753-1770. doi: 10.1042/BST20180004. Epub 2018 Dec 13. Biochem Soc Trans. 2018. PMID: 30545934 Free PMC article. Review.
-
Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy.Cancers (Basel). 2020 Mar 20;12(3):731. doi: 10.3390/cancers12030731. Cancers (Basel). 2020. PMID: 32244867 Free PMC article. Review.
-
New and Emerging Systemic Therapeutic Options for Advanced Cholangiocarcinoma.Cells. 2020 Mar 11;9(3):688. doi: 10.3390/cells9030688. Cells. 2020. PMID: 32168869 Free PMC article. Review.
References
-
- Rugo HS, Herbst RS, Liu G, Park JW, Kies MS, Steinfeldt HM, Pithavala YK, Reich SD, Freddo JL, Wilding G. Phase I trial of the oral antiangiogenesis agent AG-013736 in patients with advanced solid tumors: Pharmacokinetic and clinical results. J Clin Oncol. 2005;23:5474–5483. doi: 10.1200/JCO.2005.04.192. - DOI - PubMed
-
- Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, Qian F, Chu F, Bentzien F, Cancilla B, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10:2298–2308. doi: 10.1158/1535-7163.MCT-11-0264. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

