The art and science of study identification: a comparative analysis of two systematic reviews
- PMID: 26911333
- PMCID: PMC4766738
- DOI: 10.1186/s12874-016-0118-2
The art and science of study identification: a comparative analysis of two systematic reviews
Abstract
Background: Systematic reviews (SRs) form the foundation for guidelines and evidence-based policy in medicine and public health. Although similar systematic reviews may include non-identical sets of studies, and it is recognized that different sets of studies may lead to different conclusions, little work has been published on why SR study cohorts differ.
Methods: We took advantage of concurrent publication of two SRs on the same topic - prevention of child exposure to tobacco smoke - to understand why study cohorts differed in the two reviews. We identified all studies included in just one review, investigated validity of specified reasons for exclusions, and, using database records, explored reasons for study non-identification. We assessed review methods and discordancy, and attempted to assess whether changes in study cohorts would have changed conclusions.
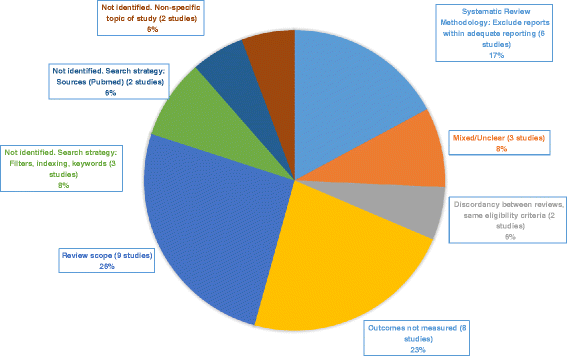
Results: Sixty-one studies were included in the two reviews. Thirty-five studies were present in just one review; of these, twenty were identified and excluded by the parallel review. Omissions were due to: review scope (9 studies, 26%), outcomes of interest not measured (8 studies, 23%), exclusion of reports with inadequate reporting (6 studies, 17%), mixed or unclear reasons (3 studies, 8%), search strategies concerning filters, tagging, and keywords (3 studies, 8%), search strategies regarding sources (PUBMED not searched) (2 studies, 6%); discordant interpretation of same eligibility criteria (2 studies, 6%), and non-identification due to non-specific study topic (2 studies, 6%). Review conclusions differed, but were likely due to differences in synthesis methods, not differences in study cohorts.
Conclusions: The process of study identification for SRs is part art and part science. While some differences are due to differences in review scope, outcomes measured, or reporting practices, others are caused by search methods or discrepancies in reviewer interpretations. Different study cohorts may or may not be a cause of differing SR results. Completeness of SR study cohorts could be enhanced by 1 - independent identification of studies by at least two reviewers, as recommended by recent guidelines, 2 - searching PUBMED with free-text keywords in addition to MEDLINE to identify recent studies, and 3 - Using validated search filters.
Figures
Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals.Cochrane Database Syst Rev. 2012 Nov 14;11(11):MR000030. doi: 10.1002/14651858.MR000030.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152285 Free PMC article. Review.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Healthcare outcomes assessed with observational study designs compared with those assessed in randomized trials.Cochrane Database Syst Rev. 2014 Apr 29;2014(4):MR000034. doi: 10.1002/14651858.MR000034.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2024 Jan 4;1:MR000034. doi: 10.1002/14651858.MR000034.pub3. PMID: 24782322 Free PMC article. Updated. Review.
Cited by
-
The Effects of Tree Nut and Peanut Consumption on Energy Compensation and Energy Expenditure: A Systematic Review and Meta-Analysis.Adv Nutr. 2023 Jan;14(1):77-98. doi: 10.1016/j.advnut.2022.10.006. Epub 2022 Dec 17. Adv Nutr. 2023. PMID: 36811596 Free PMC article. Review.
-
Understanding lifestyle self-management regimens that improve the life quality of people living with multiple sclerosis: a systematic review and meta-analysis.Health Qual Life Outcomes. 2022 Nov 25;20(1):153. doi: 10.1186/s12955-022-02046-1. Health Qual Life Outcomes. 2022. PMID: 36434609 Free PMC article. Review.
-
Technology-assisted title and abstract screening for systematic reviews: a retrospective evaluation of the Abstrackr machine learning tool.Syst Rev. 2018 Mar 12;7(1):45. doi: 10.1186/s13643-018-0707-8. Syst Rev. 2018. PMID: 29530097 Free PMC article.
-
The Metabolizable Energy and Lipid Bioaccessibility of Tree Nuts and Peanuts: A Systematic Review with Narrative Synthesis of Human and In Vitro Studies.Adv Nutr. 2023 Jul;14(4):796-818. doi: 10.1016/j.advnut.2023.03.006. Epub 2023 Mar 18. Adv Nutr. 2023. PMID: 36934832 Free PMC article. Review.
-
Tree Nut and Peanut Consumption and Risk of Cardiovascular Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Adv Nutr. 2023 Sep;14(5):1029-1049. doi: 10.1016/j.advnut.2023.05.004. Epub 2023 May 5. Adv Nutr. 2023. PMID: 37149262 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials