The effects of mycoplasma contamination upon the ability to form bioengineered 3D kidney cysts
- PMID: 25793639
- PMCID: PMC4368695
- DOI: 10.1371/journal.pone.0120097
The effects of mycoplasma contamination upon the ability to form bioengineered 3D kidney cysts
Abstract
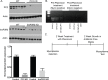
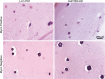
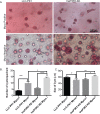
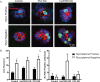
Mycoplasma contamination of cell cultures is a pervasive, often undiagnosed and ignored problem in many laboratories that can result in reduced cell proliferation and changes in gene expression. Unless contamination is specifically suspected, it is often undetected in two dimensional (2D) cultures and the resulting effects of mycoplasma contamination are rarely appreciated and can lead to incorrect conclusions. Three dimensional (3D) tissue cultures are increasingly utilized to explore tissue development and phenotype. However, 3D cultures are more complex than 2D cell cultures and require a more controlled cellular environment in order to generate structures necessary to mimic in vivo responses and are often maintained for longer time periods. Changes to the microenvironment are assumed to have a more extreme effect upon the success of 3D tissue cultures than 2D cell cultures, but the effects of mycoplasma have not been studied. To test this hypothesis, we grew 2D cell cultures and 3D tissues from pig kidney epithelial cells (LLC-PK1) that were contaminated with mycoplasma and the same stock of cells after mycoplasma removal. We did not observe an effect of mycoplasma contamination on proliferation in 2D monolayer cell culture. However, cyst formation in 3D tissues was altered, with effects upon the number, size and structure of cysts formed. These data serve to reinforce the necessity of testing cell stocks for mycoplasma contamination.
Conflict of interest statement
Figures

Similar articles
-
[Presence of Mycoplasma in laboratory cell cultures from Cordoba, Argentina].Rev Argent Microbiol. 1998 Jul-Sep;30(3):147-53. Rev Argent Microbiol. 1998. PMID: 9793145 Spanish.
-
Aptamer Cocktail to Detect Multiple Species of Mycoplasma in Cell Culture.Int J Mol Sci. 2020 May 27;21(11):3784. doi: 10.3390/ijms21113784. Int J Mol Sci. 2020. PMID: 32471128 Free PMC article.
-
Diagnosis and treatment of Mycoplasma-contaminated cell cultures.Curr Protoc Microbiol. 2006 Jan;Appendix 3:Appendix 3B. doi: 10.1002/9780471729259.mca03bs00. Curr Protoc Microbiol. 2006. PMID: 18770568
-
Routine testing of cell cultures and their products for mycoplasma contamination.Methods Mol Biol. 1997;75:305-11. doi: 10.1385/0-89603-441-0:305. Methods Mol Biol. 1997. PMID: 9276280 Review. No abstract available.
-
Operator-induced contamination in cell culture systems.Dev Biol Stand. 1991;75:193-204. Dev Biol Stand. 1991. PMID: 1794620 Review.
Cited by
-
Effects and Eradication of Mycoplasma Contamination on Patient-derived Colorectal Cancer Organoid Cultures.Cancer Res Commun. 2023 Sep 27;3(9):1952-1958. doi: 10.1158/2767-9764.CRC-23-0109. Cancer Res Commun. 2023. PMID: 37772998 Free PMC article.
-
Polycystin 2 regulates mitochondrial Ca2+ signaling, bioenergetics, and dynamics through mitofusin 2.Sci Signal. 2019 May 7;12(580):eaat7397. doi: 10.1126/scisignal.aat7397. Sci Signal. 2019. PMID: 31064883 Free PMC article.
-
Polycystin 2 is increased in disease to protect against stress-induced cell death.Sci Rep. 2020 Jan 15;10(1):386. doi: 10.1038/s41598-019-57286-x. Sci Rep. 2020. PMID: 31941974 Free PMC article.
-
Standardizing Patient-Derived Organoid Generation Workflow to Avoid Microbial Contamination From Colorectal Cancer Tissues.Front Oncol. 2022 Jan 10;11:781833. doi: 10.3389/fonc.2021.781833. eCollection 2021. Front Oncol. 2022. PMID: 35083141 Free PMC article.
References
-
- Wayner EA, Brooks CG. Induction of NKCF-like activity in mixed lymphocyte-tumor cell culture: direct involvement of mycoplasma infection of tumor cells. J Immunol. 1984;132(4):2135–42. . - PubMed
-
- Paddenberg R, Wulf S, Weber A, Heimann P, Beck LA, Mannherz HG. Internucleosomal DNA fragmentation in cultured cells under conditions reported to induce apoptosis may be caused by mycoplasma endonucleases. Eur J Cell Biol. 1996;71(1):105–19. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

