The diversity and evolution of chelicerate hemocyanins
- PMID: 22333134
- PMCID: PMC3306762
- DOI: 10.1186/1471-2148-12-19
The diversity and evolution of chelicerate hemocyanins
Abstract
Background: Oxygen transport in the hemolymph of many arthropod species is facilitated by large copper-proteins referred to as hemocyanins. Arthropod hemocyanins are hexamers or oligomers of hexamers, which are characterized by a high O2 transport capacity and a high cooperativity, thereby enhancing O2 supply. Hemocyanin subunit sequences had been available from horseshoe crabs (Xiphosura) and various spiders (Araneae), but not from any other chelicerate taxon. To trace the evolution of hemocyanins and the emergence of the large hemocyanin oligomers, hemocyanin cDNA sequences were obtained from representatives of selected chelicerate classes.
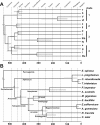
Results: Hemocyanin subunits from a sea spider, a scorpion, a whip scorpion and a whip spider were sequenced. Hemocyanin has been lost in Opiliones, Pseudoscorpiones, Solifugae and Acari, which may be explained by the evolution of trachea (i.e., taxon Apulmonata). Bayesian phylogenetic analysis was used to reconstruct the evolution of hemocyanin subunits and a relaxed molecular clock approach was applied to date the major events. While the sea spider has a simple hexameric hemocyanin, four distinct subunit types evolved before Xiphosura and Arachnida diverged around 470 Ma ago, suggesting the existence of a 4 × 6mer at that time. Subsequently, independent gene duplication events gave rise to the other distinct subunits in each of the 8 × 6mer hemocyanin of Xiphosura and the 4 × 6mer of Arachnida. The hemocyanin sequences were used to infer the evolutionary history of chelicerates. The phylogenetic trees support a basal position of Pycnogonida, a sister group relationship of Xiphosura and Arachnida, and a sister group relationship of the whip scorpions and the whip spiders.
Conclusion: Formation of a complex hemocyanin oligomer commenced early in the evolution of euchelicerates. A 4 × 6mer hemocyanin consisting of seven subunit types is conserved in most arachnids since more than 400 Ma, although some entelegyne spiders display selective subunit loss and independent oligomerization. Hemocyanins also turned out to be a good marker to trace chelicerate evolution, which is, however, limited by the loss of hemocyanin in some taxa. The molecular clock calculations were in excellent agreement with the fossil record, also demonstrating the applicability of hemocyanins for such approach.
Figures



Similar articles
-
Subunit sequences of the 4 x 6-mer hemocyanin from the golden orb-web spider, Nephila inaurata.Eur J Biochem. 2003 Aug;270(16):3432-9. doi: 10.1046/j.1432-1033.2003.03730.x. Eur J Biochem. 2003. PMID: 12899700
-
Complete hemocyanin subunit sequences of the hunting spider Cupiennius salei: recent hemocyanin remodeling in entelegyne spiders.J Biol Chem. 2002 Apr 26;277(17):14451-7. doi: 10.1074/jbc.M111368200. Epub 2002 Feb 12. J Biol Chem. 2002. PMID: 11842087
-
Diplopod hemocyanin sequence and the phylogenetic position of the Myriapoda.Mol Biol Evol. 2001 Aug;18(8):1566-73. doi: 10.1093/oxfordjournals.molbev.a003943. Mol Biol Evol. 2001. PMID: 11470848
-
Origin and evolution of arthropod hemocyanins and related proteins.J Comp Physiol B. 2002 Feb;172(2):95-107. doi: 10.1007/s00360-001-0247-7. J Comp Physiol B. 2002. PMID: 11916114 Review.
-
[Blue blood: structure and evolution of hemocyanin].Naturwissenschaften. 1989 May;76(5):206-11. doi: 10.1007/BF00627687. Naturwissenschaften. 1989. PMID: 2664531 Review. German.
Cited by
-
Upper limits to body size imposed by respiratory-structural trade-offs in Antarctic pycnogonids.Proc Biol Sci. 2017 Oct 25;284(1865):20171779. doi: 10.1098/rspb.2017.1779. Proc Biol Sci. 2017. PMID: 29070725 Free PMC article.
-
Serotonin-immunoreactivity in the ventral nerve cord of Pycnogonida--support for individually identifiable neurons as ancestral feature of the arthropod nervous system.BMC Evol Biol. 2015 Jul 10;15:136. doi: 10.1186/s12862-015-0422-1. BMC Evol Biol. 2015. PMID: 26156705 Free PMC article.
-
Myriapod haemocyanin: the first three-dimensional reconstruction of Scolopendra subspinipes and preliminary structural analysis of S. viridicornis.Open Biol. 2020 Apr;10(4):190258. doi: 10.1098/rsob.190258. Epub 2020 Apr 1. Open Biol. 2020. PMID: 32228398 Free PMC article.
-
The Globin Gene Family in Arthropods: Evolution and Functional Diversity.Front Genet. 2020 Aug 13;11:858. doi: 10.3389/fgene.2020.00858. eCollection 2020. Front Genet. 2020. PMID: 32922435 Free PMC article.
-
Molecular characterization and evolution of haemocyanin from the two freshwater shrimps Caridina multidentata (Stimpson, 1860) and Atyopsis moluccensis (De Haan, 1849).J Comp Physiol B. 2013 Jul;183(5):613-24. doi: 10.1007/s00360-013-0740-9. Epub 2013 Jan 22. J Comp Physiol B. 2013. PMID: 23338600
References
-
- Markl J, Decker H. Molecular structure of the arthropod hemocyanins. Adv Comp Environm Physiol. 1992;13:325–376. doi: 10.1007/978-3-642-76418-9_12. - DOI
-
- Van-Holde KE, Miller KI. Hemocyanins. Adv Protein Chem. 1995;47:1–81. - PubMed
-
- Markl J, Burmester T, Decker H, Savel-Niemann A, Harris JR, Süling M, Naumann U, Scheller K. Quaternary and subunit structure of Calliphora arylphorin as deduced from electron microscopy, electrophoresis, and sequence similarities with arthropod hemocyanin. J Comp Physiol B. 1992;162:665–680. doi: 10.1007/BF00301616. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

