Divergence and convergence in enzyme evolution: parallel evolution of paraoxonases from quorum-quenching lactonases
- PMID: 22069329
- PMCID: PMC3249062
- DOI: 10.1074/jbc.R111.257329
Divergence and convergence in enzyme evolution: parallel evolution of paraoxonases from quorum-quenching lactonases
Abstract
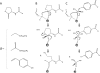
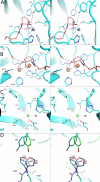
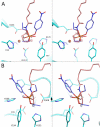
We discuss the basic features of divergent versus convergent evolution and of the common scenario of parallel evolution. The example of quorum-quenching lactonases is subsequently described. Three different quorum-quenching lactonase families are known, and they belong to three different superfamilies. Their key active-site architectures have converged and are strikingly similar. Curiously, a promiscuous organophosphate hydrolase activity is observed in all three families. We describe the structural and mechanistic features that underline this converged promiscuity and how this promiscuity drove the parallel divergence of organophosphate hydrolases within these lactonase families by either natural or laboratory evolution.
Figures

Similar articles
-
The evolutionary origins of detoxifying enzymes: the mammalian serum paraoxonases (PONs) relate to bacterial homoserine lactonases.J Biol Chem. 2013 Aug 16;288(33):23914-27. doi: 10.1074/jbc.M112.427922. Epub 2013 Jun 20. J Biol Chem. 2013. PMID: 23788644 Free PMC article.
-
Lactonases with organophosphatase activity: structural and evolutionary perspectives.Chem Biol Interact. 2010 Sep 6;187(1-3):370-2. doi: 10.1016/j.cbi.2010.01.039. Epub 2010 Feb 1. Chem Biol Interact. 2010. PMID: 20122908 Review.
-
The Structure and Function of Paraoxonase-1 and Its Comparison to Paraoxonase-2 and -3.Molecules. 2020 Dec 17;25(24):5980. doi: 10.3390/molecules25245980. Molecules. 2020. PMID: 33348669 Free PMC article. Review.
-
Enzyme Evolution: An Epistatic Ratchet versus a Smooth Reversible Transition.Mol Biol Evol. 2020 Apr 1;37(4):1133-1147. doi: 10.1093/molbev/msz298. Mol Biol Evol. 2020. PMID: 31873734
-
Structure and specificity of a quorum-quenching lactonase (AiiB) from Agrobacterium tumefaciens.Biochemistry. 2007 Oct 23;46(42):11789-99. doi: 10.1021/bi7012849. Epub 2007 Sep 28. Biochemistry. 2007. PMID: 17900178
Cited by
-
Promiscuity in the Enzymatic Catalysis of Phosphate and Sulfate Transfer.Biochemistry. 2016 Jun 7;55(22):3061-81. doi: 10.1021/acs.biochem.6b00297. Epub 2016 May 26. Biochemistry. 2016. PMID: 27187273 Free PMC article. Review.
-
AIG1 and ADTRP are atypical integral membrane hydrolases that degrade bioactive FAHFAs.Nat Chem Biol. 2016 May;12(5):367-372. doi: 10.1038/nchembio.2051. Epub 2016 Mar 28. Nat Chem Biol. 2016. PMID: 27018888 Free PMC article.
-
Structural and Biochemical Characterization of AidC, a Quorum-Quenching Lactonase with Atypical Selectivity.Biochemistry. 2015 Jul 21;54(28):4342-53. doi: 10.1021/acs.biochem.5b00499. Epub 2015 Jul 8. Biochemistry. 2015. PMID: 26115006 Free PMC article.
-
Catalytic Redundancies and Conformational Plasticity Drives Selectivity and Promiscuity in Quorum Quenching Lactonases.JACS Au. 2024 Aug 23;4(9):3519-3536. doi: 10.1021/jacsau.4c00404. eCollection 2024 Sep 23. JACS Au. 2024. PMID: 39328773 Free PMC article.
-
Toward a systems biology perspective on enzyme evolution.J Biol Chem. 2012 Jan 2;287(1):3-10. doi: 10.1074/jbc.R111.254714. Epub 2011 Nov 8. J Biol Chem. 2012. PMID: 22069330 Free PMC article. Review.
References
-
- Theobald D. L. (2010) Nature 465, 219–222 - PubMed
-
- Koonin E. V., Galperin M. Y. (2002) Sequence-Evolution-Function: Computational Approaches in Comparative Genomics, 1st Ed., Springer, New York - PubMed
-
- Kim K. M., Caetano-Anollés G. (2010) Mol. Biol. Evol. 27, 1710–1733 - PubMed
-
- Ouzounis C. A., Kunin V., Darzentas N., Goldovsky L. (2006) Res. Microbiol. 157, 57–68 - PubMed
-
- Koonin E. V. (2003) Nat. Rev. Microbiol. 1, 127–136 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

