A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms
- PMID: 19734141
- PMCID: PMC2797218
- DOI: 10.1074/jbc.R109.052340
A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms
Abstract
Together with seven ADAMTS-like proteins, the 19 mammalian ADAMTS proteases constitute a superfamily. ADAMTS proteases are secreted zinc metalloproteases whose hallmark is an ancillary domain containing one or more thrombospondin type 1 repeats. ADAMTS-like proteins resemble ADAMTS ancillary domains and lack proteolytic activity. Vertebrate expansion of the superfamily reflects emergence of new substrates, duplication of proteolytic activities in new contexts, and cooperative functions of the duplicated genes. ADAMTS proteases are involved in maturation of procollagen and von Willebrand factor, as well as in extracellular matrix proteolysis relating to morphogenesis, angiogenesis, ovulation, cancer, and arthritis. New insights into ADAMTS mechanisms indicate significant regulatory roles for ADAMTS ancillary domains, propeptide processing, and glycosylation. ADAMTS-like proteins appear to have regulatory roles in the extracellular matrix.
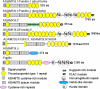
Figures

Similar articles
-
A disintegrin-like and metalloprotease (reprolysin type) with thrombospondin type 1 motifs: the ADAMTS family.Int J Biochem Cell Biol. 2004 Jun;36(6):981-5. doi: 10.1016/j.biocel.2004.01.014. Int J Biochem Cell Biol. 2004. PMID: 15094112 Review.
-
ADAMTS: a novel family of extracellular matrix proteases.Int J Biochem Cell Biol. 2001 Jan;33(1):33-44. doi: 10.1016/s1357-2725(00)00061-3. Int J Biochem Cell Biol. 2001. PMID: 11167130 Review.
-
The ADAMTS (A Disintegrin and Metalloproteinase with Thrombospondin motifs) family.Genome Biol. 2015 May 30;16(1):113. doi: 10.1186/s13059-015-0676-3. Genome Biol. 2015. PMID: 26025392 Free PMC article. Review.
-
Crystal structures of human ADAMTS-1 reveal a conserved catalytic domain and a disintegrin-like domain with a fold homologous to cysteine-rich domains.J Mol Biol. 2007 Nov 2;373(4):891-902. doi: 10.1016/j.jmb.2007.07.047. Epub 2007 Aug 2. J Mol Biol. 2007. PMID: 17897672
-
A disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) is a novel fibrillin-1-, fibrillin-2-, and heparin-binding member of the ADAMTS superfamily containing a netrin-like module.Matrix Biol. 2012 Sep-Oct;31(7-8):398-411. doi: 10.1016/j.matbio.2012.09.003. Epub 2012 Sep 23. Matrix Biol. 2012. PMID: 23010571 Free PMC article.
Cited by
-
ADAMTS6 suppresses tumor progression via the ERK signaling pathway and serves as a prognostic marker in human breast cancer.Oncotarget. 2016 Sep 20;7(38):61273-61283. doi: 10.18632/oncotarget.11341. Oncotarget. 2016. PMID: 27542224 Free PMC article.
-
Biological Significance of Local TGF-β Activation in Liver Diseases.Front Physiol. 2012 Feb 6;3:12. doi: 10.3389/fphys.2012.00012. eCollection 2012. Front Physiol. 2012. PMID: 22363291 Free PMC article.
-
ADAMTS-7 exhibits elevated expression in cartilage of osteonecrosis of femoral head and has a positive correlation with TNF- α and NF- κ B P65.Mediators Inflamm. 2015;2015:196702. doi: 10.1155/2015/196702. Epub 2015 Jan 14. Mediators Inflamm. 2015. PMID: 25653475 Free PMC article.
-
The evolutionary conservation of the A Disintegrin-like and Metalloproteinase domain with Thrombospondin-1 motif metzincins across vertebrate species and their expression in teleost zebrafish.BMC Evol Biol. 2015 Feb 15;15:22. doi: 10.1186/s12862-015-0281-9. BMC Evol Biol. 2015. PMID: 25879701 Free PMC article.
-
An anxiogenic drug, FG 7142, induced an increase in mRNA of Btg2 and Adamts1 in the hippocampus of adult mice.Behav Brain Funct. 2012 Aug 22;8:43. doi: 10.1186/1744-9081-8-43. Behav Brain Funct. 2012. PMID: 22913326 Free PMC article.
References
-
- Huxley-Jones J., Apte S. S., Robertson D. L., Boot-Handford R. P. (2005) Int. J. Biochem. Cell Biol. 37, 1838–1845 - PubMed
-
- Angerer L., Hussain S., Wei Z., Livingston B. T. (2006) Dev. Biol. 300, 267–281 - PubMed
-
- Le Goff C., Somerville R. P., Kesteloot F., Powell K., Birk D. E., Colige A. C., Apte S. S. (2006) Development 133, 1587–1596 - PubMed
-
- Levy G. G., Nichols W. C., Lian E. C., Foroud T., McClintick J. N., McGee B. M., Yang A. Y., Siemieniak D. R., Stark K. R., Gruppo R., Sarode R., Shurin S. B., Chandrasekaran V., Stabler S. P., Sabio H., Bouhassira E. E., Upshaw J. D., Jr., Ginsburg D., Tsai H. M. (2001) Nature 413, 488–494 - PubMed
-
- Zheng X., Chung D., Takayama T. K., Majerus E. M., Sadler J. E., Fujikawa K. (2001) J. Biol. Chem. 276, 41059–41063 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

