The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation
- PMID: 17640524
- PMCID: PMC2034312
- DOI: 10.1016/j.neuron.2007.06.029
The transcription factor Yin Yang 1 is essential for oligodendrocyte progenitor differentiation
Abstract
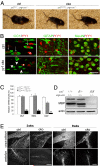
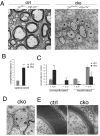
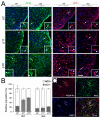
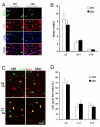
The progression of progenitors to oligodendrocytes requires proliferative arrest and the activation of a transcriptional program of differentiation. While regulation of cell cycle exit has been extensively characterized, the molecular mechanisms responsible for the initiation of differentiation remain ill-defined. Here, we identify the transcription factor Yin Yang 1 (YY1) as a critical regulator of oligodendrocyte progenitor differentiation. Conditional ablation of yy1 in the oligodendrocyte lineage in vivo induces a phenotype characterized by defective myelination, ataxia, and tremor. At the cellular level, lack of yy1 arrests differentiation of oligodendrocyte progenitors after they exit from the cell cycle. At the molecular level, YY1 acts as a lineage-specific repressor of transcriptional inhibitors of myelin gene expression (Tcf4 and Id4), by recruiting histone deacetylase-1 to their promoters during oligodendrocyte differentiation. Thus, we identify YY1 as an essential component of the transcriptional network regulating the transition of oligodendrocyte progenitors from cell cycle exit to differentiation.
Figures

Similar articles
-
Leukemia/lymphoma-related factor regulates oligodendrocyte lineage cell differentiation in developing white matter.Glia. 2012 Sep;60(9):1378-90. doi: 10.1002/glia.22356. Epub 2012 May 21. Glia. 2012. PMID: 22615173
-
Myelin transcription factor 1 (Myt1) modulates the proliferation and differentiation of oligodendrocyte lineage cells.Mol Cell Neurosci. 2004 Jan;25(1):111-23. doi: 10.1016/j.mcn.2003.10.001. Mol Cell Neurosci. 2004. PMID: 14962745
-
The DYT6 Dystonia Protein THAP1 Regulates Myelination within the Oligodendrocyte Lineage.Dev Cell. 2017 Jul 10;42(1):52-67.e4. doi: 10.1016/j.devcel.2017.06.009. Dev Cell. 2017. PMID: 28697333 Free PMC article.
-
Transcriptional and post-transcriptional control of CNS myelination.Curr Opin Neurobiol. 2010 Oct;20(5):601-7. doi: 10.1016/j.conb.2010.05.005. Epub 2010 Jun 16. Curr Opin Neurobiol. 2010. PMID: 20558055 Review.
-
Cytology and lineage of NG2-positive glia.J Neurocytol. 2002 Jul-Aug;31(6-7):457-67. doi: 10.1023/a:1025735513560. J Neurocytol. 2002. PMID: 14501216 Review.
Cited by
-
Myelin repair strategies: a cellular view.Curr Opin Neurol. 2008 Jun;21(3):278-83. doi: 10.1097/WCO.0b013e3282fd1875. Curr Opin Neurol. 2008. PMID: 18451710 Free PMC article. Review.
-
CLC-2 is a positive modulator of oligodendrocyte precursor cell differentiation and myelination.Mol Med Rep. 2018 Mar;17(3):4515-4523. doi: 10.3892/mmr.2018.8439. Epub 2018 Jan 17. Mol Med Rep. 2018. PMID: 29344669 Free PMC article.
-
Targeting histone deacetylases for the treatment of disease.J Cell Mol Med. 2009 May;13(5):826-52. doi: 10.1111/j.1582-4934.2008.00571.x. Epub 2008 Nov 3. J Cell Mol Med. 2009. PMID: 19175682 Free PMC article. Review.
-
YY1 Is Required for Posttranscriptional Stability of SOX2 and OCT4 Proteins.Cell Reprogram. 2017 Aug;19(4):263-269. doi: 10.1089/cell.2017.0002. Epub 2017 Jul 6. Cell Reprogram. 2017. PMID: 28682643 Free PMC article.
-
The Transcriptional Activator Krüppel-like Factor-6 Is Required for CNS Myelination.PLoS Biol. 2016 May 23;14(5):e1002467. doi: 10.1371/journal.pbio.1002467. eCollection 2016 May. PLoS Biol. 2016. PMID: 27213272 Free PMC article.
References
-
- Alvarez-Salas LM, Benitez-Hess ML, Dipaolo JA. YY-1 and c-Jun transcription factors participate in the repression of the human involucrin promoter. Int J Oncol. 2005;26:259–266. - PubMed
-
- Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. - PubMed
-
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

