Epidermolysis bullosa simplex-type mutations alter the dynamics of the keratin cytoskeleton and reveal a contribution of actin to the transport of keratin subunits
- PMID: 14668478
- PMCID: PMC363056
- DOI: 10.1091/mbc.e03-09-0687
Epidermolysis bullosa simplex-type mutations alter the dynamics of the keratin cytoskeleton and reveal a contribution of actin to the transport of keratin subunits
Abstract
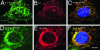
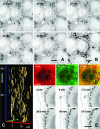
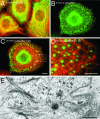
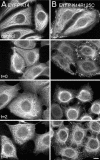
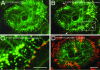
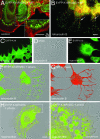
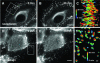
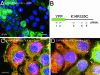
Dominant keratin mutations cause epidermolysis bullosa simplex by transforming keratin (K) filaments into aggregates. As a first step toward understanding the properties of mutant keratins in vivo, we stably transfected epithelial cells with an enhanced yellow fluorescent protein-tagged K14R125C mutant. K14R125C became localized as aggregates in the cell periphery and incorporated into perinuclear keratin filaments. Unexpectedly, keratin aggregates were in dynamic equilibrium with soluble subunits at a half-life time of <15 min, whereas filaments were extremely static. Therefore, this dominant-negative mutation acts by altering cytoskeletal dynamics and solubility. Unlike previously postulated, the dominance of mutations is limited and strictly depends on the ratio of mutant to wild-type protein. In support, K14R125C-specific RNA interference experiments resulted in a rapid disintegration of aggregates and restored normal filaments. Most importantly, live cell inhibitor studies revealed that the granules are transported from the cell periphery inwards in an actin-, but not microtubule-based manner. The peripheral granule zone may define a region in which keratin precursors are incorporated into existing filaments. Collectively, our data have uncovered the transient nature of keratin aggregates in cells and offer a rationale for the treatment of epidermolysis bullosa simplex by using short interfering RNAs.
Figures


Similar articles
-
Functional testing of keratin 14 mutant proteins associated with the three major subtypes of epidermolysis bullosa simplex.Exp Dermatol. 2003 Aug;12(4):472-9. doi: 10.1034/j.1600-0625.2002.120416.x. Exp Dermatol. 2003. PMID: 12930305
-
Reduction in keratin aggregates in epidermolysis bullosa simplex keratinocytes after pretreatment with trimethylamine N-oxide.Exp Dermatol. 2016 Mar;25(3):229-30. doi: 10.1111/exd.12821. Epub 2015 Sep 15. Exp Dermatol. 2016. PMID: 26264477 No abstract available.
-
Epidermolysis bullosa simplex: a disorder of keratin.Lancet. 1992 Jan 4;339(8784):29-30. Lancet. 1992. PMID: 1370336 No abstract available.
-
Posttranslational modifications of keratins and their associated proteins as therapeutic targets in keratin diseases.Curr Opin Cell Biol. 2023 Dec;85:102264. doi: 10.1016/j.ceb.2023.102264. Epub 2023 Nov 3. Curr Opin Cell Biol. 2023. PMID: 37925932 Review.
-
Keratotic lesions in epidermolysis bullosa simplex with mottled pigmentation.J Am Acad Dermatol. 2005 Jan;52(1):172-3. doi: 10.1016/j.jaad.2004.07.046. J Am Acad Dermatol. 2005. PMID: 15627110 Review. No abstract available.
Cited by
-
Complementary roles of specific cysteines in keratin 14 toward the assembly, organization, and dynamics of intermediate filaments in skin keratinocytes.J Biol Chem. 2015 Sep 11;290(37):22507-19. doi: 10.1074/jbc.M115.654749. Epub 2015 Jul 27. J Biol Chem. 2015. PMID: 26216883 Free PMC article.
-
Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease.Am J Hum Genet. 2006 Mar;78(3):510-9. doi: 10.1086/500850. Epub 2006 Jan 19. Am J Hum Genet. 2006. PMID: 16465624 Free PMC article.
-
Defining the properties of the nonhelical tail domain in type II keratin 5: insight from a bullous disease-causing mutation.Mol Biol Cell. 2005 Mar;16(3):1427-38. doi: 10.1091/mbc.e04-06-0498. Epub 2005 Jan 12. Mol Biol Cell. 2005. PMID: 15647384 Free PMC article.
-
Keratins as an Inflammation Trigger Point in Epidermolysis Bullosa Simplex.Int J Mol Sci. 2021 Nov 18;22(22):12446. doi: 10.3390/ijms222212446. Int J Mol Sci. 2021. PMID: 34830328 Free PMC article. Review.
-
Identification of novel principles of keratin filament network turnover in living cells.Mol Biol Cell. 2004 May;15(5):2436-48. doi: 10.1091/mbc.e03-09-0707. Epub 2004 Mar 5. Mol Biol Cell. 2004. PMID: 15004233 Free PMC article.
References
-
- Amann, K.J., and Pollard, T.D. (2001). The Arp2/3 complex nucleates actin filament branches from the sides of pre-existing filaments. Nat. Cell Biol. 3, 306-310. - PubMed
-
- Andra, K., Kornacker, I., Jorgl, A., Zorer, M., Spazierer, D., Fuchs, P., Fischer, I., and Wiche, G. (2003). Plectin-isoform-specific rescue of hemidesmosomal defects in plectin (-/-) keratinocytes. J. Investig. Dermatol. 120, 189-197. - PubMed
-
- Anton-Lamprecht, I., and Schnyder, U.W. (1982). Epidermolysis bullosa herpetiformis Dowling-Meara. Report of a case and pathomorphogenesis. Dermatologica 164, 221-235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

