Researchers at the National Eye Institute (NEI) have improved a crucial step in the production of retinal pigment epithelium (RPE), a tissue they grow in the lab from patient blood cells and are testing in a clinical trial as treatment for age-related macular degeneration (AMD). Their technique combines electrochemical cell measurements enhanced by mathematical modeling to directly assess epithelial cell membranes and tissue barrier health. The RPE is located in the back of the eye and helps keep the retina’s light-sensing photoreceptors healthy.
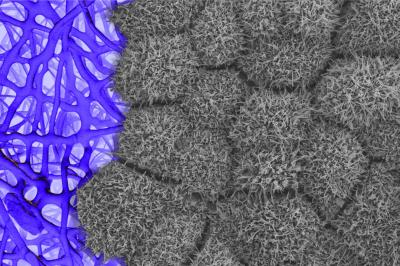
Seen here is the apical surface of patient-derived retinal pigment epithelium (grey) growing on a biodegradable scaffold. Credit Kapil Bharti, National Eye Institute.
Epithelial tissues maintain a protective barrier between organs’ internal and external environments, ensuring normal function. For example, epithelial tissues cover the gastrointestinal tract, lungs, skin, eye, and countless other surfaces of the body. Importantly, disruption of epithelial integrity via cell damage is associated with many diseases, such as celiac disease, cystic fibrosis, diabetes, and AMD. RPE breakdown is often the first step in AMD pathogenesis.
“Our technique, which we call 3-probe electrochemical impedance spectroscopy, or 3 P-EIS, provides additional physiologic parameters that are useful in manufacturing RPE tissues used in cell therapies,” said Colby Lewallen, Ph.D., a postdoctoral fellow at the NEI. “In addition, 3 P-EIS provides greater resolution data, with respect to epithelial function in drug testing and disease modeling protocols.”
Colby published a report about the technique in Cell Physiology with co-senior authors Kapil Bharti, Ph.D., from the NEI, and Craig Forest, Ph.D., a professor of bioengineering at the Georgia Institute of Technology. The authors plan to use 3 P-EIS for quality control in an ongoing phase I/IIa clinical trial of patient-derived RPE for AMD, one of the most common causes of blindness in the U.S.
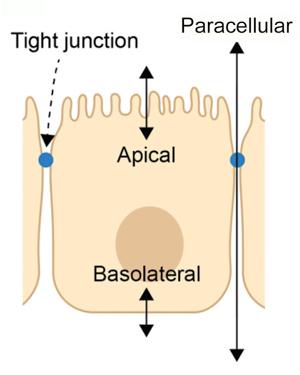
Side-view illustration of an RPE cell showing the three main pathways where ion transport is regulated.
Lewallen and colleagues used an electric circuit “breadboard” to simulate the well-known electrochemical properties of RPE tissue, testing nearly 1,000 possible configurations of the apical, basolateral, the paracellular pathways, and the extracellular environments. Using measurements from their tissue simulator, they developed an algorithm to directly measure each pathway’s contribution to the charge across living tissues.
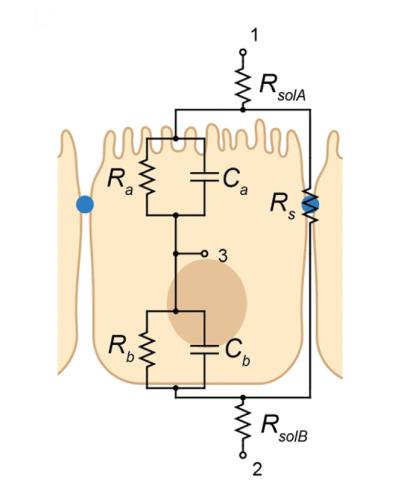
Illustration showing a cross-section of epithelial tissue and additional pathways of ion transport that are measurable with 3 P-EIS. With electrodes placed at nodes 1, 2, and 3, 3 P-EIS can predict the apical resistance and capacitance (Ra and Ca), the basolateral resistance and capacitance (Rb and Cb), and the shunt (Rs) properties of epithelial tissues.
The scientists tested their new technique using stem cell-derived RPE in a device called an Ussing chamber, which enables them to apply a stimulus to the living tissue and test the effect of drugs while simultaneously recording the electrochemical changes across each membrane and the entire tissue. Collected data are then fed to the mathematical model to extract precise values of changes that occur at different cell membranes.
According to the authors, 3 P-EIS provides direct measurements of the main electrochemical pathways, eliminating the need for time-consuming and resource-intensive follow-up experiments. Going forward, they are optimizing the algorithm and making it more accessible and easier to use by other researchers in the field. They hope their technique will be adopted as the new comprehensive assay for future drug discovery, clinical trials, and basic science research.
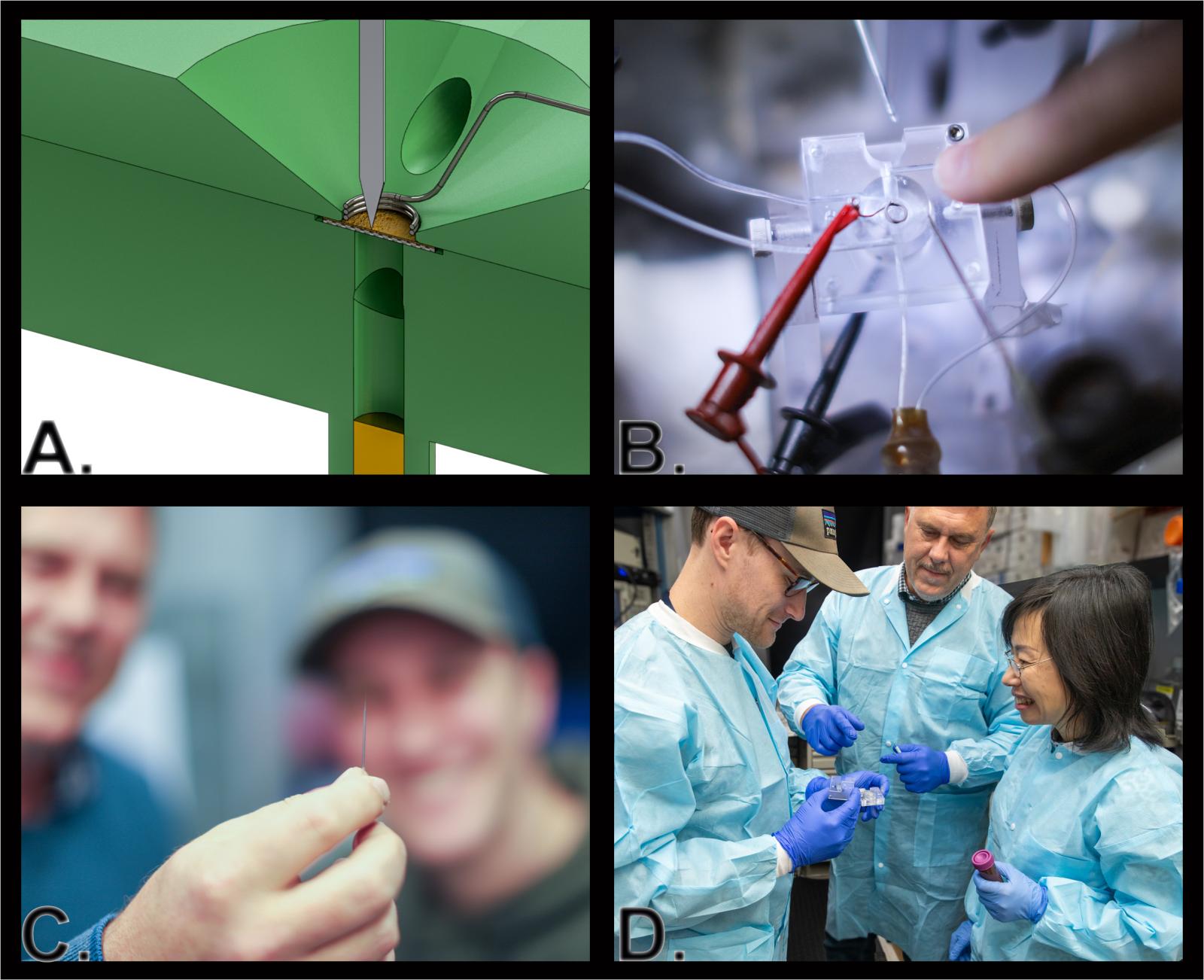
A) Cross-sectional illustration of a modified Ussing chamber, used to measure the electrical properties of epithelial tissues. B) Photo of a modified Ussing chamber. C) Glass pipette used to measure charge inside individual epithelial cells. The pipette’s tip is too small to be seen with the naked eye. D) NEI researchers Colby Lewallen, Ph.D. (left) Arvydas Maminishkis, M.D., Ph.D., and Qin Wan, Ph.D. readying an Ussing chamber.
Reference
Lewallen CF, Chien A, Maminishkis A, et al. A biologically validated mathematical model for decoding epithelial apical, basolateral, and paracellular electrical properties. Am J Physiol Cell Physiol. Dec 1 2023;325(6):C1470-c1484. doi:10.1152/ajpcell.00200.2023.