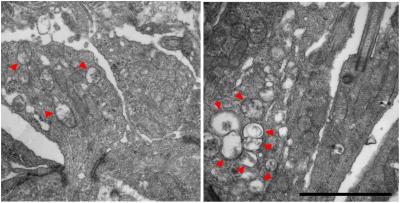
Mitochondrial structural alterations are shown at early stages before photoreceptor degeneration in rd1 retinas. Red arrowheads indicate abnormal mitochondria with swollen cristae at postnatal days 8 (left) and 10. Credit: Anand Swaroop, Ph.D.
Molecular and cellular changes in rod photoreceptors are detectable in a mouse model of retinal degeneration several days prior to observable morphological changes, according to researchers at the National Eye Institute. The findings, published in Human Molecular Genetics, could point to new therapeutic targets for retinal degenerative diseases such as retinitis pigmentosa (RP) and age-related macular degeneration (AMD).
“Prior to this study, we lacked knowledge of the precise cellular events and molecular cues that precede the onset of pathology. We now have a better understanding of the natural history of neuronal cell death in degenerative disease,” said the study’s lead investigator, Anand Swaroop, Ph.D., senior investigator and chief of the NEI Neurobiology Neurodegeneration and Repair Laboratory, part of the National Institutes of Health.
The results provide a temporal framework for understanding the role of mitochondria-related, metabolic, and calcium signaling pathways in early stages preceding the onset of retinal neurodegeneration.
“Interventions targeting components in such pathways can serve as a potential treatment strategy regardless of the specific genetic mutation involved,” Swaroop said.
Rod and cone photoreceptors are responsible for initiating the visual cycle, the crucial process that converts light into electrical signals sent from the eye to the brain. Photoreceptor dysfunction and death lead to vision loss since the retina cannot regenerate these cells. Generally, rod cells die before cones in RP and AMD. Understanding what causes rod cell death is important for designing treatment for retinal neurodegeneration.
Using the widely studied retinal neurodegeneration mouse model Pde6brd1/rd1, the researchers investigated molecular and cellular events that might initiate and/or trigger rod cell death by applying an integrated multi-omics approach. That is, they used state-of-the-art technologies to detect proteins (proteomics), metabolites (metabolomics) and mRNA to see which genes actually get expressed (transcriptomics) at different stages of neonatal development. They analyzed proteins and metabolites in the retina as a whole and mRNA in rods.
As early as postnatal day 10, notable cell structural changes were evident in the model. Photoreceptors are long tube-like structures divided into inner and outer segments. Mouse models had a shortening of their rod photoreceptors’ outer segments, the region where the visual cycle occurs. The outer nuclear layer also had become thinner.
Where previous studies have focused on disease progression at or after onset of rod death (after postnatal day 10), transcriptome profiling of rods at days 2-6 in this new research shows major and highly specific abnormalities in expression of genes associated with mitochondria and metabolism.
Furthermore, the gene expression trends suggest that mutant photoreceptors never undergo normal development despite their normal appearance in early stages. Mitochondria and components of related metabolic and calcium signaling pathways demonstrate significant changes in early developing rods. At postnatal day 6, for example, high levels of mitochondrial stress were evident prior to signs of structural and functional photoreceptor degeneration.
Importantly, the research showed enhanced mitochondrial turnover and defects in many calcium-regulated proteins including calmodulin, which is a sensor of intracellular calcium levels. The results indicate that metabolic imbalance and mitochondrial abnormalities are related to the abnormal calcium signaling that precedes rod death. Calcium signaling drives mitochondrial and metabolic changes and is likely the mediator of the cellular response in retinal neurodegeneration.
The results suggest that reducing calcium levels via drug therapy may be one possibility for slowing retinal degeneration. Another might be to address mitochondrial stress by fine tuning the glycolysis-tricarboxylic acid cycle with dietary supplementation.
The study was supported by the NEI Intramural Research Program.
References:
Jiang K, Mondal AK, Adlakha YK, Gumerson J, Aponte A, Gieser L, Kim JW, Boleda A, Brooks MJ, Nellissery J, Fox DA, Balaban R, Covian R, Swaroop A. “Multi-omics analyses reveal early metabolic imbalance and mitochondrial stress in neonatal photoreceptors leading to cell death in Pde6brd1/rd1 mouse model of retinal degeneration.” Published January 24, 2022 in Human Molecular Genetics. https://doi.org/10.1093/hmg/ddac013
##
This press release describes a basic research finding. Basic research increases our understanding of human behavior and biology, which is foundational to advancing new and better ways to prevent, diagnose, and treat disease. Science is an unpredictable and incremental process— each research advance builds on past discoveries, often in unexpected ways. Most clinical advances would not be possible without the knowledge of fundamental basic research. To learn more about basic research, visit https://www.nih.gov/news-events/basic-research-digital-media-kit.
NEI leads the federal government’s research on the visual system and eye diseases. NEI supports basic and clinical science programs to develop sight-saving treatments and address special needs of people with vision loss. For more information, visit https://www.nei.nih.gov.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit https://www.nih.gov/.
NIH…Turning Discovery Into Health®