Regulated N-glycosylation controls chaperone function and receptor trafficking
- PMID: 39509507
- PMCID: PMC7617332
- DOI: 10.1126/science.adp7201
Regulated N-glycosylation controls chaperone function and receptor trafficking
Abstract
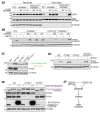
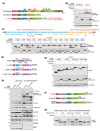
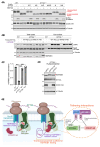
One-fifth of human proteins are N-glycosylated in the endoplasmic reticulum (ER) by two oligosaccharyltransferases, OST-A and OST-B. Contrary to the prevailing view of N-glycosylation as a housekeeping function, we identified an ER pathway that modulates the activity of OST-A. Genetic analyses linked OST-A to HSP90B1, an ER chaperone for membrane receptors, and CCDC134, an ER luminal protein. During its translocation into the ER, an N-terminal peptide in HSP90B1 templates the assembly of a translocon complex containing CCDC134 and OST-A that protects HSP90B1 during folding, preventing its hyperglycosylation and degradation. Disruption of this pathway impairs WNT and IGF1R signaling and causes the bone developmental disorder osteogenesis imperfecta. Thus, N-glycosylation can be regulated by specificity factors in the ER to control cell surface receptor signaling and tissue development.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
CCDC134 controls TLR biogenesis through the ER chaperone Gp96.J Exp Med. 2025 Mar 3;222(3):e20240825. doi: 10.1084/jem.20240825. Epub 2024 Dec 10. J Exp Med. 2025. PMID: 39656203 Free PMC article.
-
Suppression of Dad1 induces cardiomyocyte death by weakening cell adhesion.Am J Physiol Cell Physiol. 2025 Jan 1;328(1):C95-C106. doi: 10.1152/ajpcell.00509.2024. Epub 2024 Nov 29. Am J Physiol Cell Physiol. 2025. PMID: 39611549
-
Modulating Endoplasmic Reticulum Chaperones and Mutant Protein Degradation in GABRG2(Q390X) Associated with Genetic Epilepsy with Febrile Seizures Plus and Dravet Syndrome.Int J Mol Sci. 2024 Apr 23;25(9):4601. doi: 10.3390/ijms25094601. Int J Mol Sci. 2024. PMID: 38731820 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
A review of the mammalian unfolded protein response.Biotechnol Bioeng. 2011 Dec;108(12):2777-93. doi: 10.1002/bit.23282. Epub 2011 Aug 9. Biotechnol Bioeng. 2011. PMID: 21809331 Free PMC article. Review.
References
-
- Zielinska DF, Gnad F, Wiśniewski JR, Mann M. Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell. 2010;141:897–907. - PubMed
-
- Gagneux P, Hennet T, Varki A. In: Essentials of Glycobiology. Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Mohnen D, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, editors. Cold Spring Harbor Laboratory Press; Cold Spring Harbor (NY): 2022. Biological Functions of Glycans. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

