Chikungunya Virus Vaccines: A Review of IXCHIQ and PXVX0317 from Pre-Clinical Evaluation to Licensure
- PMID: 39292392
- PMCID: PMC11530495
- DOI: 10.1007/s40259-024-00677-y
Chikungunya Virus Vaccines: A Review of IXCHIQ and PXVX0317 from Pre-Clinical Evaluation to Licensure
Abstract
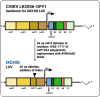
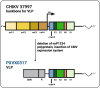
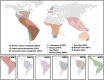
Chikungunya virus is an emerging mosquito-borne alphavirus that causes febrile illness and arthritic disease. Chikungunya virus is endemic in 110 countries and the World Health Organization estimates that it has caused more than 2 million cases of crippling acute and chronic arthritis globally since it re-emerged in 2005. Chikungunya virus outbreaks have occurred in Africa, Asia, Indian Ocean islands, South Pacific islands, Europe, and the Americas. Until recently, no specific countermeasures to prevent or treat chikungunya disease were available. To address this need, multiple vaccines are in human trials. These vaccines use messenger RNA-lipid nanoparticles, inactivated virus, and viral vector approaches, with a live-attenuated vaccine VLA1553 and a virus-like particle PXVX0317 in phase III testing. In November 2023, the US Food and Drug Administration (FDA) approved the VLA1553 live-attenuated vaccine, which is marketed as IXCHIQ. In June 2024, Health Canada approved IXCHIQ, and in July 2024, IXCHIQ was approved by the European Commission. On August 13, 2024, the US FDA granted priority review for PXVX0317. The European Medicine Agency is considering accelerated assessment review of PXVX0317, with potential for approval by both agencies in 2025. In this review, we summarize published data from pre-clinical and clinical trials for the IXCHIQ and PXVX0317 vaccines. We also discuss unanswered questions including potential impacts of pre-existing chikungunya virus immunity on vaccine safety and immunogenicity, whether long-term immunity can be achieved, safety in children, pregnant, and immunocompromised individuals, and vaccine efficacy in people with previous exposure to other emerging alphaviruses in addition to chikungunya virus.
© 2024. The Author(s).
Conflict of interest statement
Whitney C. Weber, Daniel N. Streblow, and Lark L. Coffey have no conflicts of interest that are directly relevant to the content of this article.
Figures

Similar articles
-
Safety and immunogenicity of a live-attenuated chikungunya virus vaccine in endemic areas of Brazil: interim results of a double-blind, randomised, placebo-controlled phase 3 trial in adolescents.Lancet Infect Dis. 2025 Jan;25(1):114-125. doi: 10.1016/S1473-3099(24)00458-4. Epub 2024 Sep 5. Lancet Infect Dis. 2025. PMID: 39243794 Clinical Trial.
-
Antibody persistence and safety of a live-attenuated chikungunya virus vaccine up to 2 years after single-dose administration in adults in the USA: a single-arm, multicentre, phase 3b study.Lancet Infect Dis. 2024 Dec;24(12):1383-1392. doi: 10.1016/S1473-3099(24)00357-8. Epub 2024 Aug 12. Lancet Infect Dis. 2024. PMID: 39146946 Clinical Trial.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Chikungunya vaccine development, challenges, and pathway toward public health impact.Vaccine. 2024 Dec 2;42(26):126483. doi: 10.1016/j.vaccine.2024.126483. Epub 2024 Oct 29. Vaccine. 2024. PMID: 39467413 Review.
References
-
- WHO. Chikungunya virus. Available from: https://www.who.int/health-topics/chikungunya#tab=tab_1. Accessed 19 Jul 2024.
-
- Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. Trans Royal Soc Trop Med Hyg. 1955;49:28. - PubMed
-
- Kuhn RJ. Togaviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields’ virology. 5th ed. New York: Lippincott, Williams and Wilkins; 2007. p. 1001–22.
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM142619/GM/NIGMS NIH HHS/United States
- R01AI125902/Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases
- U19AI142790/Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases
- T32GM142619/Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases

