Stenotrophomonas maltophilia uses a c-di-GMP module to sense the mammalian body temperature during infection
- PMID: 39231185
- PMCID: PMC11404848
- DOI: 10.1371/journal.ppat.1012533
Stenotrophomonas maltophilia uses a c-di-GMP module to sense the mammalian body temperature during infection
Abstract
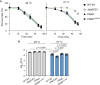
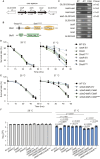
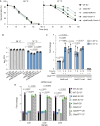
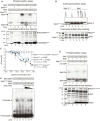
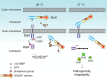
The body temperature of Warm-blooded hosts impedes and informs responses of bacteria accustomed to cooler environments. The second messenger c-di-GMP modulates bacterial behavior in response to diverse, yet largely undiscovered, stimuli. A long-standing debate persists regarding whether a local or a global c-di-GMP pool plays a critical role. Our research on a Stenotrophomonas maltophilia strain thriving at around 28°C, showcases BtsD as a thermosensor, diguanylate cyclase, and effector. It detects 37°C and diminishes c-di-GMP synthesis, resulting in a responsive sequence: the periplasmic c-di-GMP level is decreased, the N-terminal region of BtsD disengages from c-di-GMP, activates the two-component signal transduction system BtsKR, and amplifies sod1-3 transcription, thereby strengthening the bacterium's pathogenicity and adaptation during infections in 37°C warm Galleria mellonella larvae. This revelation of a single-protein c-di-GMP module introduces unrecognized dimensions to the functional and structural paradigms of c-di-GMP modules and reshapes our understanding of bacterial adaptation and pathogenicity in hosts with a body temperature around 37°C. Furthermore, the discovery of a periplasmic c-di-GMP pool governing BtsD-BtsK interactions supports the critical role of a local c-di-GMP pool.
Copyright: © 2024 Wang et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Thermoregulation of Biofilm Formation in Burkholderia pseudomallei Is Disrupted by Mutation of a Putative Diguanylate Cyclase.J Bacteriol. 2017 Feb 14;199(5):e00780-16. doi: 10.1128/JB.00780-16. Print 2017 Mar 1. J Bacteriol. 2017. PMID: 27956524 Free PMC article.
-
LotS/LotR/Clp, a novel signal pathway responding to temperature, modulating protease expression via c-di-GMP mediated manner in Stenotrophomonas maltophilia FF11.Microbiol Res. 2018 Sep;214:60-73. doi: 10.1016/j.micres.2018.05.014. Epub 2018 May 19. Microbiol Res. 2018. PMID: 30031482
-
Cyclic di-GMP Signaling in Bacillus subtilis Is Governed by Direct Interactions of Diguanylate Cyclases and Cognate Receptors.mBio. 2020 Mar 10;11(2):e03122-19. doi: 10.1128/mBio.03122-19. mBio. 2020. PMID: 32156823 Free PMC article.
-
High-specificity local and global c-di-GMP signaling.Trends Microbiol. 2021 Nov;29(11):993-1003. doi: 10.1016/j.tim.2021.02.003. Epub 2021 Feb 24. Trends Microbiol. 2021. PMID: 33640237 Review.
-
Sensory Perception in Bacterial Cyclic Diguanylate Signal Transduction.J Bacteriol. 2022 Feb 15;204(2):e0043321. doi: 10.1128/JB.00433-21. Epub 2021 Oct 4. J Bacteriol. 2022. PMID: 34606374 Free PMC article. Review.
References
-
- Rothfork JM, Timmins GS, Harris MN, Chen X, Lusis AJ, Otto M, et al.. Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: new role for the NADPH oxidase in host defense. Proc Natl Acad Sci U S A. 2004;101(38):13867–72. Epub 2004/09/09. doi: 10.1073/pnas.0402996101 ; PubMed Central PMCID: PMC518845. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

