Halogenated Analogs to Natural A-Type Proanthocyanidins: Evaluation of Their Antioxidant and Antimicrobial Properties and Possible Application in Food Industries
- PMID: 39125027
- PMCID: PMC11314616
- DOI: 10.3390/molecules29153622
Halogenated Analogs to Natural A-Type Proanthocyanidins: Evaluation of Their Antioxidant and Antimicrobial Properties and Possible Application in Food Industries
Abstract
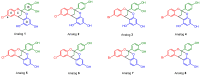
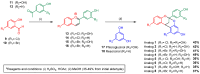
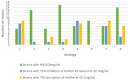
A description of new antimicrobial agents suitable for food industries has become necessary, and natural compounds are being considered as promising sources of new active derivatives to be used with the aim of improving food safety. We have previously described desirable antimicrobial and antibiofilm activities against foodborne bacteria by analogs to A-type proanthocyanidins (PACs) with a nitro (NO2) group at carbon 6 of the A-ring. We report herein the synthesis of eight additional analogs with chloro and bromo atoms at the A-ring and the systematic study of their antimicrobial and antioxidant activities in order to evaluate their possible application as biocides or food preservatives, as well as to elucidate new structure-activity relationships. The results from this study show that halogenated analogs to natural A-type proanthocyanidins rise above the nitro derivatives previously reported in their antimicrobial activities. Gram-positive bacteria are the most sensitive to all the analogs and combinations assayed, showing MICs from 10 to 50 μg/mL in most cases, as well as reductions in biofilm formation and the disruption of preformed biofilms of at least 75%. Some structure-activity relationships previously described have also been corroborated. Analogs with just one OH group at the B-ring show better antimicrobial activities than those with two OH groups, and those analogs with two or three OH groups in the whole structure are more active than those with four OH groups. In addition, the analogs with two OH groups at the B-ring and chloro at the A-ring are the most effective when antibiofilm activities are studied, especially at low concentrations.
Keywords: A-type proanthocyanidin analogs; antibiofilm activity; antimicrobial activity; antioxidant activity; flavylium chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Synthesis of Analogs to A-Type Proanthocyanidin Natural Products with Enhanced Antimicrobial Properties against Foodborne Microorganisms.Molecules. 2023 Jun 19;28(12):4844. doi: 10.3390/molecules28124844. Molecules. 2023. PMID: 37375401 Free PMC article.
-
Synthesis and Evaluation of Antimicrobial and Antibiofilm Properties of A-Type Procyanidin Analogues against Resistant Bacteria in Food.J Agric Food Chem. 2018 Mar 7;66(9):2151-2158. doi: 10.1021/acs.jafc.8b00535. Epub 2018 Feb 21. J Agric Food Chem. 2018. PMID: 29464945
-
Synthesis and Isolation of Phenol- and Thiol-Derived Epicatechin Adducts Prepared from Avocado Peel Procyanidins Using Centrifugal Partition Chromatography and the Evaluation of Their Antimicrobial and Antioxidant Activity.Molecules. 2024 Jun 17;29(12):2872. doi: 10.3390/molecules29122872. Molecules. 2024. PMID: 38930937 Free PMC article.
-
Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms.Phytomedicine. 2017 Dec 15;37:14-26. doi: 10.1016/j.phymed.2017.10.021. Epub 2017 Nov 23. Phytomedicine. 2017. PMID: 29174600 Review.
-
Natural and Synthetic Halogenated Amino Acids-Structural and Bioactive Features in Antimicrobial Peptides and Peptidomimetics.Molecules. 2021 Dec 6;26(23):7401. doi: 10.3390/molecules26237401. Molecules. 2021. PMID: 34885985 Free PMC article. Review.
References
-
- CDC Database Data from National Outbreak Reporting System (NORS) of Centers for Disease Control and Prevention (CDC) [(accessed on 1 December 2021)];2021 Available online: https://wwwn.cdc.gov/norsdashboard/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical