Relevance of genetic testing in the gene-targeted trial era: the Rostock Parkinson's disease study
- PMID: 39087914
- PMCID: PMC11292909
- DOI: 10.1093/brain/awae188
Relevance of genetic testing in the gene-targeted trial era: the Rostock Parkinson's disease study
Abstract
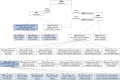
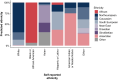
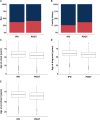
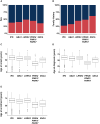
Estimates of the spectrum and frequency of pathogenic variants in Parkinson's disease (PD) in different populations are currently limited and biased. Furthermore, although therapeutic modification of several genetic targets has reached the clinical trial stage, a major obstacle in conducting these trials is that PD patients are largely unaware of their genetic status and, therefore, cannot be recruited. Expanding the number of investigated PD-related genes and including genes related to disorders with overlapping clinical features in large, well-phenotyped PD patient groups is a prerequisite for capturing the full variant spectrum underlying PD and for stratifying and prioritizing patients for gene-targeted clinical trials. The Rostock Parkinson's disease (ROPAD) study is an observational clinical study aiming to determine the frequency and spectrum of genetic variants contributing to PD in a large international cohort. We investigated variants in 50 genes with either an established relevance for PD or possible phenotypic overlap in a group of 12 580 PD patients from 16 countries [62.3% male; 92.0% White; 27.0% positive family history (FH+), median age at onset (AAO) 59 years] using a next-generation sequencing panel. Altogether, in 1864 (14.8%) ROPAD participants (58.1% male; 91.0% White, 35.5% FH+, median AAO 55 years), a PD-relevant genetic test (PDGT) was positive based on GBA1 risk variants (10.4%) or pathogenic/likely pathogenic variants in LRRK2 (2.9%), PRKN (0.9%), SNCA (0.2%) or PINK1 (0.1%) or a combination of two genetic findings in two genes (∼0.2%). Of note, the adjusted positive PDGT fraction, i.e. the fraction of positive PDGTs per country weighted by the fraction of the population of the world that they represent, was 14.5%. Positive PDGTs were identified in 19.9% of patients with an AAO ≤ 50 years, in 19.5% of patients with FH+ and in 26.9% with an AAO ≤ 50 years and FH+. In comparison to the idiopathic PD group (6846 patients with benign variants), the positive PDGT group had a significantly lower AAO (4 years, P = 9 × 10-34). The probability of a positive PDGT decreased by 3% with every additional AAO year (P = 1 × 10-35). Female patients were 22% more likely to have a positive PDGT (P = 3 × 10-4), and for individuals with FH+ this likelihood was 55% higher (P = 1 × 10-14). About 0.8% of the ROPAD participants had positive genetic testing findings in parkinsonism-, dystonia/dyskinesia- or dementia-related genes. In the emerging era of gene-targeted PD clinical trials, our finding that ∼15% of patients harbour potentially actionable genetic variants offers an important prospect to affected individuals and their families and underlines the need for genetic testing in PD patients. Thus, the insights from the ROPAD study allow for data-driven, differential genetic counselling across the spectrum of different AAOs and family histories and promote a possible policy change in the application of genetic testing as a routine part of patient evaluation and care in PD.
Keywords: GBA1; LRRK2; Parkinson’s disease; genetic factors; genetic testing; next-generation sequencing.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
V.S., C.B., J.J.P., F.C., S.S, H.G., M.O., X.B., N.A., K.K.K., A.R., P.B., were or still are employees of CENTOGENE GmbH. A.W. provides consultancy services around research projects for CENTOGENE GmbH. C.K. and N.B. are medical advisors to CENTOGENE GmbH. The other authors report no competing interests.
Figures
Similar articles
-
Parkinson's disease variant detection and disclosure: PD GENEration, a North American study.Brain. 2024 Aug 1;147(8):2668-2679. doi: 10.1093/brain/awae142. Brain. 2024. PMID: 39074992 Free PMC article.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
The effectiveness of school-based family asthma educational programs on the quality of life and number of asthma exacerbations of children aged five to 18 years diagnosed with asthma: a systematic review protocol.JBI Database System Rev Implement Rep. 2015 Oct;13(10):69-81. doi: 10.11124/jbisrir-2015-2335. JBI Database System Rev Implement Rep. 2015. PMID: 26571284
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Hemophilia B.2000 Oct 2 [updated 2024 Jun 6]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2000 Oct 2 [updated 2024 Jun 6]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301668 Free Books & Documents. Review.
Cited by
-
Clinical genetic testing in Parkinson's disease should become part of routine patient care.Brain. 2024 Aug 1;147(8):2595-2597. doi: 10.1093/brain/awae181. Brain. 2024. PMID: 39087915
-
Are rare heterozygous SYNJ1 variants associated with Parkinson's disease?NPJ Parkinsons Dis. 2024 Oct 25;10(1):201. doi: 10.1038/s41531-024-00809-9. NPJ Parkinsons Dis. 2024. PMID: 39455605 Free PMC article.
-
The LRRK2 p.L1795F variant causes Parkinson's disease in the European population.Res Sq [Preprint]. 2024 Sep 20:rs.3.rs-4772543. doi: 10.21203/rs.3.rs-4772543/v1. Res Sq. 2024. PMID: 39372927 Free PMC article. Preprint.
References
-
- Lange LM, Gonzalez-Latapi P, Rajalingam R, et al. . Nomenclature of genetic movement disorders: Recommendations of the international Parkinson and movement disorder society task force—An update. Mov Disord. 2022;37:905–935. - PubMed
-
- Vollstedt E-J, Schaake S, Lohmann K, et al. . Embracing monogenic Parkinson’s disease: The MJFF global genetic PD cohort. Mov Disord. 2023;38:286–303. - PubMed

