Efficacy and Safety of Osilodrostat in Managing Cushing's Syndrome: A Systematic Review and Meta-Analysis
- PMID: 39086571
- PMCID: PMC11288521
- DOI: 10.4103/ijem.ijem_260_23
Efficacy and Safety of Osilodrostat in Managing Cushing's Syndrome: A Systematic Review and Meta-Analysis
Abstract
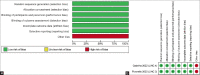
No meta-analysis has holistically analysed and summarized the efficacy and safety of osilodrostat, a novel dual 11β-hydroxylase (cytochrome P450 family 11 subfamily B member 1 [CYP11B1]) and 18-hydroxylase (aldosterone synthase, CYP11B2) inhibitor in managing Cushing's syndrome (CS). We undertook this meta-analysis to address this knowledge gap. Electronic databases were searched for randomized controlled trials (RCTs) involving patients with CS receiving osilodrostat in the intervention arm. The primary outcome was to evaluate changes in urine free cortisol (UFC) levels. Secondary outcomes were to evaluate alterations in cortisol levels, androgen levels, mineralocorticoid levels, and adverse events. From initially screened 109 articles, data from 2 RCTs involving 144 patients was analysed. After 8-12 weeks of therapy, the odds of achieving a normal 24-hour UFC was higher in patients receiving oslidrostat as compared to placebo. [odds ratio (OR) 21.94 (95% CI: 8.53-56.43); P < 0.00001; I2 = 0%]. The occurrence of adverse events [OR 1.35 (95% CI: 0.52-3.53); P = 0.54; I2 = 0%; low heterogeneity (LH); High certainty of evidence (HCE)], serious adverse events (SAEs) [OR 1.32 (95% CI: 0.30-5.79); P = 0.72; I2 = 0%; LH; HCE], adrenal insufficiency [OR 5.38 (95% CI: 0.91-31.78); P = 0.06; I2 = 0%; LH; HCE], headache [OR 0.98 (95% CI: 0.35-2.76); P = 0.97; I2 = 0%; LH; HCE], hyperandrogenism [OR 3.68 (95% CI: 0.59-22.80); P = 0.16; I2 = 0%; LH; HCE] and deaths [OR 0.32 (95% CI: 0.01-8.00); P = 0.48; I2 = 0%; LH; HCE] was comparable among the groups. The occurrence of nausea [OR 4.25 (95% CI: 1.26-14.30); P = 0.02; I2 = 0%; LH] and arthralgia [OR 6.54 (95% CI: 1.64-26.13); P = 0.008; I2 = 0%; LH; HCE] was significantly higher in the osilodrostat group as compared to placebo. Osilodrostat has good efficacy and safety in CS and was well tolerated over 48 weeks of use.
Keywords: Adrenal; cushing’s disease; cushing’s syndrome; osilodrostat; pituitary.
Copyright: © 2024 Indian Journal of Endocrinology and Metabolism.
Conflict of interest statement
There are no conflicts of interest.
Figures

Similar articles
-
Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): a multicentre phase III study with a double-blind, randomised withdrawal phase.Lancet Diabetes Endocrinol. 2020 Sep;8(9):748-761. doi: 10.1016/S2213-8587(20)30240-0. Epub 2020 Jul 27. Lancet Diabetes Endocrinol. 2020. PMID: 32730798 Clinical Trial.
-
A multicenter, phase 2 study to evaluate the efficacy and safety of osilodrostat, a new 11β-hydroxylase inhibitor, in Japanese patients with endogenous Cushing's syndrome other than Cushing's disease.Endocr J. 2020 Aug 28;67(8):841-852. doi: 10.1507/endocrj.EJ19-0617. Epub 2020 May 1. Endocr J. 2020. PMID: 32378529 Clinical Trial.
-
Osilodrostat, a potent oral 11β-hydroxylase inhibitor: 22-week, prospective, Phase II study in Cushing's disease.Pituitary. 2016 Apr;19(2):138-48. doi: 10.1007/s11102-015-0692-z. Pituitary. 2016. PMID: 26542280 Free PMC article. Clinical Trial.
-
Clinical Utility of Osilodrostat in Cushing's Disease: Review of Currently Available Literature.Drug Des Devel Ther. 2023 Apr 27;17:1303-1312. doi: 10.2147/DDDT.S315359. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37143705 Free PMC article. Review.
-
Treatment of Cushing's syndrome with osilodrostat: practical applications of recent studies with case examples.Pituitary. 2022 Dec;25(6):795-809. doi: 10.1007/s11102-022-01268-2. Epub 2022 Aug 24. Pituitary. 2022. PMID: 36002784 Free PMC article. Review.
References
-
- Martino M, Aboud N, Lucchetti B, Salvio G, Arnaldi G. Osilodrostat oral tablets for adults with Cushing's disease. Expert Rev Endocrinol Metab. 2022;17:99–109. - PubMed
-
- Bertagna X, Pivonello R, Fleseriu M, Zhang Y, Robinson P, Taylor A, et al. LCI699, a potent 11β- hydroxylase inhibitor, normalizes urinary cortisol in patients with Cushing's disease: Results from a multicenter, proof-of-concept study. J Clin Endocrinol Metab. 2014;99:1375–83. - PubMed
-
- Creemers SG, Feelders RA, de Jong FH, Franssen GJH, de Rijke YB, van Koetsveld PM, et al. Osilodrostat is a potential novel steroidogenesis inhibitor for the treatment of Cushing syndrome: An in vitro study. J Clin Endocrinol Metab. 2019;104:3437–49. - PubMed
-
- Pivonello R, Fleseriu M, Newell-Price J, Bertagna X, Findling J, Shimatsu A, et al. Efficacy and safety of osilodrostat in patients with Cushing's disease (LINC 3): A multicentre phase III study with a double-blind, randomised withdrawal phase. Lancet Diabetes Endocrinol. 2020;8:748–61. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
